



|
Role of Gamma-irradiated Basil (Ocimumbasilicum) in the Alleviation of Heart Toxicity Induced by Arsenic in Rats
Aishah H. Ghazwani1*, Nadia N. Osman1,2, Khadijah S. Balamash1 |
|
1Department of Biochemistry, King Abdulaziz University, Jeddah, Saudi Arabia. 2 Department of Food Irradiation Research, National Centre for Radiation Research and Technology, Cairo, Egypt. |
ABSTRACT
Exposure to pollution from the environment is a very important factor that impacts on health. Arsenic is considered one of these pollutants and it has harmful effects on various organs including the heart. This study aimed to assess the effect of raw and irradiated basil on the damage of the heart in rats exposed to arsenic. Albino rats (n=32) were divided into four groups as follows "Control" group received distilled water, "As" group received arsenic(10mg/kg), "As+Basil" group received raw basil(400mg/kg) along with arsenic (10mg/kg) and "As+Irr. basil" group received 400mg/kg of irradiated basil (10kGy) along with arsenic (10mg/kg). To estimate the effect of gamma-irradiation on the antioxidant properties in basil, Fourier-transform infrared (FTIR) spectroscopy was used. This analysis revealed an increase in the antioxidants compounds in irradiated basil as compared to raw basil. Rats exposed to arsenic showed a significant increase in serum lipid profile, cardiac enzymes as well as increasing the heart oxidative stress with a decline in their antioxidants. The administration of raw or gamma-irradiated basil leaves to arsenic exposed rats has significantly reduced the accumulation of lipids and enzymes in serum accompanied by an improvement in antioxidant and oxidative stress of the heart. In conclusion, our findings showed that raw or irradiated basil has a therapist effect on cardiac damage prompted by arsenic.
Key Words: Basil; Gamma irradiation; Arsenic; Cardiotoxicity; Antioxidants.
INTRODUCTION
Arsenic is naturally present in the soil, rock, deep wells, and oceans [1, 2]. Arsenic is introduced either naturally or anthropogenically into the environment; the natural processes like volcanic eruptions, weathering of sedimentary rocks, and soil erosion while the anthropogenic processes include smelting (separation of metal from rock) for industrial purposes [3]. Arsenic ranks first on the priority list of hazardous substances, over other toxic metals such as cadmium, lead, and mercury, according to the Agency for Toxic Substances and Disease Registry (ATSDR) Drug Priority List 2017 [4]. It can transform into persistent metallic compounds with high toxicity [5]. The deleterious effects of arsenic exposure that reported, it induced disturbances of heart, lipid homeostasis, and the content of oxidants and antioxidants [6, 7]. Toxicity with arsenic can cause defect in cellular respiration due to glycolysis [8]. The managed of these side effects may be effective with treating by alternative medicine.
In general, herbal plants have been used for years as supplements and additionally to treat illnesses [9].
Basil (Ocimum basilicumis) is one of the herbal plants are recognized in alternative medicine, that is known to be rich in secondary metabolites and could be useful in the production of natural drugs [10]. Basil has an ability as a protective effect against inflammation, oxidative, ulcer, high blood sugar and lipid, mutagenesis, hypertension, and cardiovascular diseases [11, 12]. However, concerns about plant contamination are challenges to the safe use of the herbs. Where the contamination in natural plant products occurs by a host of environmental, agricultural factors, coupled with harvesting and storage techniques [13, 14]. According to the Food and Drug Administration, microbial contaminants such as Salmonella, Klebsiella, Bacillus, Enterobacter, Staphylococcus aureus and, Pseudomonas are commonly found in herbs [15]. If untreated, herbs will cause serious effects on health and the economy [16]. Thus, to ensure safety, decontamination effective and safe method should apply.
The irradiation method with gamma-ray is reported to be suitable for microbial decontamination from the dried plant with no adverse effect, and the dose of its recommended is 9–13 kGy [17]. Irradiation is used as an alternative to conventional treatment include heating, cooling, high-pressure, cleaning, pesticide, and chemical fumigation techniques [18]. It inhibits the species reproduction cycle by destroying nuclear material primarily (prevention of further biological development and reproduction), whilst other methods are measured principally by the mortality of species [19].
The present study aimed to evaluate the effect of gamma radiation on the basil plant and evaluate the protective effect of raw and irradiated basil on arsenic-induced cardiac toxicity.
MATERIALS AND METHODS
Chemicals
Sodium arsenate (Na2HAsO4) was obtained from BDH Chemicals Ltd. Company (UK).
Plant
Five-hundred grams of dried leaves of basil were purchased from the local traditional market in Jeddah, Saudi Arabia.
Two hundred and fifty grams of basil leaves were transferred into polyethylene bags and treated with 10 KGy of gamma-rays, using a 60Co source at a dose rate of 4.75 KGy/ h at the National Centre for Radiation Research and Technology (NCRRT), Cairo, Egypt.
Every two hundred and fifty grams of raw and irradiated dried leaves basil (Ocimum basilicum) was soaked in 3L of distilled water with stirring for 1 hour without boiling to be filled each part of a leaf with water. Then boil it for 30 min. The obtained extract was filtered first over cheesecloth then over Whatman® filter paper and the filtrate was collected, then water was removed using a rotary evaporator (HS-2005S) at 60 ºC to obtain a semi-dry extract. Then these extract transferred into the freeze dryer vacuum overnight to obtain the full-dry extract. It is then kept at 4 °C and used when starting the experiment with dissolving it in an appropriate amount of distilled water according to the used dose.
A small number of powdered leaf samples was mixed with potassium bromide (KBr) in a mortar. Press the mixed powder to form a pellet in the pressing machine 5000-10000 pound-force per square inch(psi). The formed pellet loaded in the FTIR spectroscope JASCO model, with a Scan range from 400 to 4000 cm-1. Analysis of the spectral data was performed using OriginPro software.
Animals
This study was conducted on 32 adult female albino rats weighing between 150 and 200 g from the Faculty of Pharmacy, King Abdulaziz University, KSA. The animals were housed in cages and received normal rat chow and tap water ad libitum in a constant environmental (room temperature 28±2 °C, room humidity 60±5%) with a 12 h light and 12 h dark cycle. The animals were kept under observation for two weeks before starting the experiments. Animal procedures were performed following the Ethics Committee approval number 254-18 of the Unit of Biomedical Ethics, Research Ethics Committee at the Faculty of Medicine, King Abdulaziz University and following recommendations for the proper care and use of laboratory animals.
Experimental Design
Thirty-two rats were divided into four groups of eight rats each as follows:
‘Control’ group: given only distilled water.
‘As’ group: rats received orally a sodium arsenate (10mg/kg body weight/day) [20].
‘As+Basil’ group: rats received orally a sodium arsenate (10mg/kg body weight/day) [20] and a water extract of raw basil (400mg/kg body weight/day) [21].
‘As+Irr. basil’ group: rats received orally a sodium arsenate (10mg/kg body weight/day) [20] and a water extract of irradiated basil (400mg/kg body weight/day) [21].
At the end of the experimental period (5 weeks), rats fasted overnight before scarification. Blood sample withdrawn from each rat's retro-orbital plexus by a heparinized capillary tube, then centrifuged to separate serum at 3600 rmp for 15 min, which stored for serum biochemical analyses at -40ºC. Rats were sacrificed after blood sampling and the heart was quickly removed, then stored for tissue biochemical analyses at -40ºC.
Biochemical analysis
The serum triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), creatine kinase (CK), and lactate dehydrogenase (LDH) were identified using the kits with a CAT. NO. 10724, 10017, 10018, 10094, EC 2.7.3.2, and EC 1.1.1.27, respectively (HUMAN company, Germany) while serum very-low-density lipoprotein cholesterol (VLDL) was calculated by dividing the triglyceride value by 5 [22].
For tissue analysis, the heart was homogenized and estimated each of malondialdehyde (MDA), catalase (CAT), reduced glutathione (GSH), superoxide dismutase (SOD) according to the kits with a CAT. NO. ab118970, ab83464, ab138881, and ab65354, respectively (Abcam company, UK), while for the protein concentration was estimated in tissues by used PierceTM bicinchoninic acid (BCA) protein kit with a CAT. NO. 23225 (Thermo Fisher Scientific company, US).
Statistical analysis
The data of each group were analyzed using MegaStat 9.4 (an add-in for Excel). The data were expressed as arithmetic mean and standard deviation of the mean (SD). Differences between groups were analyzed for parametric parameters using one-way variance analysis (ANOVA), the least significant difference equation (LSD). A P value below or equal to 0.05 was considered significant.
RESULTS
FTIR analysis
From the FTIR of dried leaves of raw and irradiated showed the presence of characteristic bands at 3384 cm-1 (O-H groups), 2921 cm-1 (C-H groups), 1634 cm-1 (C=O groups),1383 cm-1 (N-O groups) and 1036 cm-1 (C-N group) as in Table1 and Figure 1.
Table 1: FTIR peak values and functional groups of basil as raw and irradiated
|
Peak value (cm-1) |
Bond type |
Functional groups |
|
3384 |
O-H |
Hydroxyl |
|
2921 |
C-H |
Aromatics |
|
1634 |
C=O |
Carboxylic acid |
|
1383 |
N-O |
Nitro compounds |
|
1036 |
C-N |
Aliphatic amines |
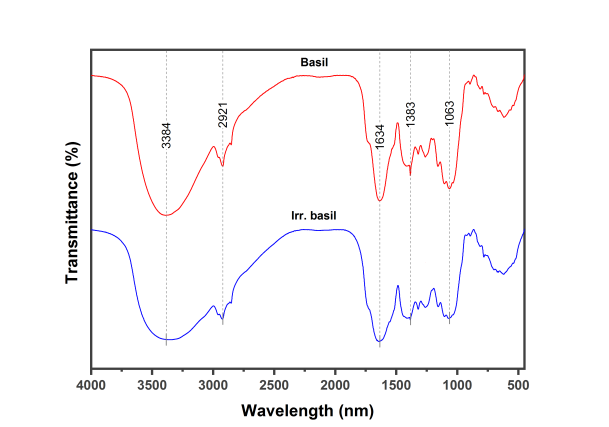
Figure 1: FTIR spectra of basil as raw and irradiated
Lipid profile
Table 2 showed the effect of raw or irradiated basil on serum lipid profile in rats exposed to arsenic. In the arsenic group, there was a highly significant elevated in TG, TC, LDL, and VLDL accompanied by a highly significant reduction in HDL level as compared with a control group. Co-treatment with arsenic and raw or irradiated basil was contributed to corrected lipid profile to a near-normal level by decreased the TG, TC, LDL and VLDL level as compared to that of the arsenic-treated group, while showed significantly increased in HDL level only in rats that treated by irradiated basil along with arsenic as compared to those of rats treated with arsenic alone.
Table 2: Effect of raw and irradiated basil extracts on serum lipid profile of rats exposed to arsenic.
|
|
TG (mg/dl) |
TC (mg/dl) |
HDL (mg/dl) |
LDL (mg/dl) |
VLDL (mg/dl) |
|
Control |
57.805 ± 5.790 |
53.014 ± 3.995 |
32.547 ± 3.757 |
30.126 ± 2.614 |
11.561 ± 1.158 |
|
As |
84.385 ± 6.360*** |
75.808 ± 5.143*** |
26.060 ± 3.479*** |
39.157 ± 5.831*** |
16.877 ± 1.272*** |
|
As + Basil |
67.162 ± 8.612**### |
57.722 ± 5.232### |
29.284 ± 3.014* |
33.295 ± 2.945## |
13.432 ± 1.722**### |
|
As + Irr. basil |
62.373 ± 5.974### |
59.713 ± 4.074*### |
30.059 ± 3.663# |
33.566 ± 2.767## |
12.475 ± 1.195### |
Values are the mean of 8 observation ± SD.
Significant different from control value at P >0.05*, 0.01**, 0.001***
Significant different from As at value at P >0.05#, 0.01##, 0.001###
Enzymes activity
The effect of administration of aqueous extracts of raw and irradiated basil on serum levels of CK and LDH in rats exposed to arsenic are summarized in Table 3. Rats, which were exposed to arsenic, showed a significant raised in serum enzyme activities compared to a control group. Administration of raw or irradiated basil along with arsenic reduced these enzyme levels as compared to an arsenic group. Regarding the effect of gamma-irradiation on the therapeutic effect of basil, no significant difference in arsenic groups administrated with either raw or irradiated basil at LDH level, but the CK level showed decreased in rats given arsenic and irradiated basil as compared with the group that given arsenic and raw basil.
Oxidative stress and antioxidant defense
The effect of administration of aqueous extracts of raw and irradiated basil on lipid peroxidation (MDA) and antioxidants (CAT, GSH, and SOD) in the heart of rats treated with arsenic are summarized in Table 4. Rats exposed to arsenic showed highly significant elevated in MDA levels accompanied by a significant decrease in glutathione and antioxidant enzymes in cardiac tissue as compared to a control group. However, the administration of raw or irradiated basil along with arsenic alleviated the effects of arsenic and resulted in a significant increase in CAT, GSH and SOD levels with a significantly decreased MDA level when compared to an arsenic group.
Table 3: Effect of basil extracts on enzymes activities in the serum of rats exposed to arsenic
|
|
CK (U/I) |
LDH (U/I) |
|
Control |
84.798 ± 7.957 |
578.558 ± 40.225 |
|
As |
185.423 ± 8.449*** |
874.645 ± 51.627*** |
|
As + Basil |
133.457 ± 4.809***### |
703.187 ± 46.782***### |
|
As + Irr. basil |
124.095 ± 4.453***###^ |
698.131 ± 44.989***### |
Each value represents the mean of 8 rats ± SD
Significant different from control value at P> 0.001***
Significant different from As at value at P >0.001###
Significant different from As + Basil at a value at P >0.05^
Table 4: Effect of basil extracts on cardiac oxidative stress and antioxidants of rats exposed to arsenic
|
|
MDA (nmol/g tissue) |
CAT (nmol/min/mg of protein) |
GSH (µM/g tissue) |
SOD (Activity (inhibition rate%) |
|
Control |
2.367 ±0.412 |
0.018 ±0.0007 |
71.657 ±5.014 |
87.889 ±10.014 |
|
As |
3.588 ± 0.165*** |
0.009 ± 0.0005*** |
48.587 ± 4.400*** |
58.237 ± 7.973*** |
|
As + Basil |
3.060 ± 0.204***## |
0.012 ± 0.0008***# |
58.475 ± 7.316***### |
65.659 ± 8.462*** |
|
As + Irr. basil |
2.844 ± 0.447**### |
0.014 ± 0.0033***### |
61.837 ± 5.400***### |
73.746 ± 6.902**## |
Each value represents the mean of 8 rats ± SD
Significant different from control value at P> 0.01**, 0.001***
Significant different from As at value at P >0.05#, 0.01##, 0.001###
DISCUSSION
FTIR analysis
FTIR is a technique that identifies chemical bonds or functional groups in the sample by producing an infrared absorption spectrum. The produced spectra indicate the contents of the sample [23].
To identify the qualitative content, the spectrum produced from the absorption of infrared radiation in the sample will be compared with reference spectra in computer databases or the spectrum obtained from a known material. The spectrum matches will contribute to identifying the sample components [24]. While the quantities of content will be identified from the size of peaks in the spectrum [25].
In the present study, the FTIR spectroscopy was used to determine chemical bonds in raw and irradiated samples of basil based on the peak values. The results obtained indicated the presence of the following functional group. Hydroxyl groups (O-H) at 3384 cm-1 which indicates the presence of hydroxy compounds such as phenolic acids and flavonoids that are natural antioxidants. An increase in the peak volume after irradiation has been observed, indicating an increase in the amount of these compounds. These findings are in agreement with El Shahat, El-Shennawy and Abd El-Megid [26] who demonstrated that gamma-radiation contributes to increased total phenolic contents and total flavonoid content in basil. Regarding improved antioxidant activity, after gamma irradiation processing, Adamo et al. [27] believed that the gamma-rays can break chemical bonds in polyphenols, thus release phenols that have soluble properties. Hydrocarbon groups (C-H) at 2921 cm-1 which indicates aromatic compounds found in essential oils of basil. Lawtrakul, Inthajak, and Toochinda [28] found that the major aromatic compounds in the essential oil of basil are eugenol, estragole, and methyl eugenol. Carbonyl groups (C=O) at 1634 cm-1 indicate the presence of the carboxylic acid in basil such as glutamic acid and aspartic acid. Our findings are in agreement with Bleiziffer et al. [29] who reported that the dominant amino acids identified in basil were glutamic acid and aspartic acid. Nitro groups (N-O) at 1383 cm-1 of nitro compounds. Kadhim, Sosa and Hameed [30] have demonstrated that basil contains a nitro compound such as (1,2,4-Triazole, 4- [N-(2- 1 hydroxyethyl)-N-nitro] amino). Aliphatic amine groups (C-N) at 1036 cm-1 which in turn revealed to the presence of alanine, leucine, and glycine. Bleiziffer et al. [29] analyzed amino acids in basil extract and they confirmed the existence of these aliphatic amines.
Lipid profile
The liver has a key role in xenobiotic detoxification and protection against chemical toxicity [31]. Besides this, it is a major organ that controls whole-body lipid homeostasis [32]. The increased gathering of arsenic could be responsible for liver dysfunction resulting in disruption of serum and cellular lipid levels, which in turn leads to account for the genesis of cardiovascular diseases [33, 34].
In the present study, it was demonstrated that arsenic exposure could cause dyslipidemia by disrupting lipid homeostasis through increased levels of TG, TC, LDL, and VLDL, while reduced HDL. Similar results were obtained by several studies on arsenic at dose 3 mg/ kg body weight [35, 36] and with dose (100 ppm) [37] in drinking water of rats.
The increase in the cholesterol level may be linked to arsenic negative impacts which cause mostly increased activity of the enzyme 3-hydroxy-3-methyl-glutaryl CoA (HMG-CoA) reductase [38]. HMG-CoA reductase catalyzes the production of mevalonate from HMG-CoA, in which the HMG-CoA reductase reaction is the rate-limiting step in cholesterol synthesis [39]. Thus, the increase in HMG-CoA reductase activity results in excessive cholesterol production and accumulation. Also, arsenic causes a decrease in lecithin cholesterol acyltransferase (LCAT) [38]. The LCAT enzyme helps transport cholesterol from the blood and tissues through a process called cholesterol esterification, which formation of cholesterol esters from free cholesterol in nascent HDL. This allows the HDL particles to mature and then HDL transports the cholesterol to the liver. Once in the liver, the cholesterol removed from the body [40, 41]. While the increase in the triglycerides level in arsenic-treated rats may be due to arsenic-induced elevation of plasma free fatty acid (FFA) concentration [42]. This exceeds might lead to increased hepatic FFA uptake, in turn, provides an immediate substrate for triglyceride synthesis [43].
In our study, basil supplementation as raw or irradiated contributed to improving the lipid homeostasis in arsenic-treated rats. This effect was studied by many researchers, Touiss et al. [44] studied the effect of phenolic extract from basil at a dose of 200 mg/kg body weight in hyperlipidemic mice and found that treatment with the extract decreased total cholesterol and LDL with suppressed the high plasma triglycerides. Al-Sahhaf [45] reported that basil aqueous extract at a dose level of 0.2mg/ml improves triglycerides and cholesterol in sodium hypochlorite-induced biochemical alterations in rabbits. Moreover, Chandra et al. [46] observed that the treatment of diabetic rats with aqueous extracts of basil 10 mg/kg body weight caused a lowering of total cholesterol and triglycerides accompanied by an increase in the level of HDL.
Basil’s ability to restore the distorted lipid homeostasis may due to its contents such as flavonoids, beta carotene, and tannins. Flavonoids and beta carotene could inhibit HMG-CoA reductase which known to functions as a catalyst in the formation of cholesterol. HMG-CoA reductase inhibition leads to reduced cholesterol concentration in hepatocytes and therefore upregulation of LDL receptors [47]. The increasing hepatic availability of LDL receptors leading to the accelerated clearance of LDL and VLDL [48]. The increased clearance of LDL and VLDL is responsible for the reduction of LDL cholesterol and triglyceride levels in plasma. Flavonoids can also improve (LCAT) function, which leads to raises in serum HDL levels [49]. Furthermore, tannins can reduce cholesterol and LDL by increasing rates of cholesterol metabolism into bile acids and increase the excretion of bile acids through feces [50].
Enzymes activity
As known, the levels of the cardiac biomarkers (CK and LDH) in blood symbolizing the condition and health of the heart, the high levels of these enzymes indicating heart injured [51]. Although heavy metal toxicity is not primarily targeted the heart tissue [52], it was found an association between arsenic and cardiovascular diseases [53].
According to the present data, it is evident that arsenic exposure accompanied by increased cardiac biomarkers (CK and LDH). These results are in line with other research findings [7, 54, 55]. These increased could be due to possible leakage of enzymes across the damaged membrane of heart muscle cells. Oyagbemi et al. [56] reported that arsenic caused necrosis of myocardial cells, which leads to a loss of plasma membrane integrity, thereby its leads to leakage cardiac enzymes into the bloodstream [57].
The administration of raw or irradiated basil reduced cardiac injury. This finding is consistent with the previous study, which reported that the administration of the methanolic extract of basil at dose 150mg/kg body weight caused a lower level of cardiac enzymes in the rat model of myocardial infarction [58]. This may due to possessing basil a high content of magnesium and potassium that improving the health of the cardiovascular system [59]. Also, basil contains omega-3 fatty acids [29], which reported that they have the potential for protecting and stabilizing cardiac tissue against arsenic-induced cardiotoxicity in vivo and in vitro[60].
Oxidative stress and antioxidant defense
"oxidative stress is associated with a disturbance in the prooxidant-antioxidant balance in favor of the pro-oxidant" [61]. Oxidative stress can be caused through the metabolism of toxicants that leads to generated excessive free radicals such as reactive oxygen species (ROS), which may interact with cellular macromolecules, including lipids, DNA, and proteins, resulting in changed of structural and functional integrity of cells [62].
In the present study, the administration of arsenic caused significantly increased MDA accompanied by decreased CAT, SOD, and GSH in cardiac tissue. These results are in agreement with the findings by Li et al. [55] who reported that exposure to arsenic significantly decreased the activities of enzymatic and nonenzymic antioxidants (SOD, CAT, glutathione peroxidase (GPx), and GSH) while the level of MDA was significantly increased in the heart. These findings can be explained by the effect of arsenic which destroys the structure and functions of mitochondrial to produce an excess of electrons that can transform oxygen into the free radicals (superoxide anion) [63]. These free radicals are necessary but in trace amounts, for normal reactions such as in a defending against infectious agents and redox homeostasis [64]. In the case of free radical’s excess, these oxidants induce a chain reaction, resulting in lipid oxidation or lipid peroxidation, disrupting redox signaling and trigger molecular damage while damaging antioxidant defense systems [65].
In contrast, this study finds that basil has decreased lipid peroxidation (MDA) and enhanced the antioxidant CAT, SOD, and GSH. These results are consistent with Arya et al. [66] that demonstrated basil reduced the level of MDA and restored the activities of antioxidant enzymes CAT, SOD, and GPx in heart tissue in myocardial necrosis experimental model. Basil is a good source of scavenging free radicals due to it contains compounds have antioxidant properties such as vitamins (E, C, and β-Carotene) [59], essential oils (linalool, estragole, and methyl cinnamate) [67], phenolic compounds (flavonoids and phenolic acids) [68]. So, the co-administration of arsenic with raw or irradiated basil results in amelioration of oxidative stress by antioxidants.
CONCLUSION
Arsenic affects the heart by causing a disturbance in lipid levels and heart enzyme activities as well as cardiac lipid peroxidation and antioxidants. Basil is poss therapeutic effects on cardiotoxicity. Treated basil with gamma-ray is noting induces increased antioxidant quantity.
ACKNOWLEDGEMENT
The authors wish to express their deepest gratitude to Wejdan Anbari from the department of Chemistry, King Abdulaziz University for her suggestion to work on FIIR and help us do it to get the best possible results.
Disclosure statement
No potential conflict of interest was reported by the authors.
REFERENCES