



|
Improvement of Glucose uptake in 3T3-L1adipocyte Cells by Aqueous and Hydro-alcoholic Extract of Prosopis farcta
Mohadeseh Mehrizi1, 2, Hossein Nazari1, Hadi Amrollahi3* |
|
1 Department of Biochemistry, Semnan University of Medical Sciences, Semnan, Iran. 2 Research Committee Student, Semnan University of Medical Sciences, Semnan, Iran. 3 Department of Medical Mycology and Parasitology, Semnan University of Medical Sciences, Semnan, Iran. |
ABSTRACT
Background & objectives: Chemical drugs are controlling diabetes mellitus and not effective for the absolute cure. By emphasis on traditional medicine and considering the antioxidant property of Prosopis farcta containing the phenol and flavonoid components, this study was conducted to evaluate the effect of hydro-alcoholic and aqueous extract of P. farcta on the glucose uptake level in 3T3-L1 adipocytes. Methods: Recent in vitro study with cells which were grown with Dulbecco's Modified Eagle Medium (DMEM) and were divided into control group, positive control, negative control group by preventing glucose uptake stimulation with 1 µM Cytochalacin D and treatment groups containing glucose uptake stimulation with aqueous and hydroalcoholic extracts of P. farcta at a different dosage. To measure the amount of glucose uptake by cells, the instruction of the Abcam glucose uptake kit was used. Results: Doses of 160 µg/mL of hydro-alcoholic and 800 µg/ml of the aqueous extract of P. farcta have the maximum amount of 2- deoxyglucose uptake in adipocyte cells (p<0/001). Between 800 µg/mL dosage comparison, the aqueous extracts had the most pronounced effect (p<0/001). Interpretation & conclusions: Findings showed that both aqueous and hydroalcoholic extract of P. farcta in a dose-dependent manner have been effective in increasing the level of glucose uptakes into the 3T3-L1 adipocytes. However, future studies must find possible molecular pathways associated with P. farcta action.
Key Words: Prosopis farcta, glucose uptake, 3T3-L1 Adipocyte cells, Insulin.
INTRODUCTION
Diabetes mellitus (DM) is prevalent in most of the developed and developing countries. It is a chronic disorder of carbohydrates, proteins, and fats metabolism due to relative or absolute insulin deficiency [1]. This metabolic disorder leads to chronic hyperglycemia and in the long-term, damages the tissues and organs, and if not diagnosed early or under lack of continuous control, life is threatened. World Health Organization (WHO) estimates that approximately, 3 million people die due to DM annually [2]. Today, 5% of the world's population is suffering from DM and within the next 25 years, it will be one of the main causes of fatal and debilitating factors for human beings [3]. According to the recent International Diabetes Federation (IDF) survey, almost 170 million people around the world were suffering from this disease in 2010, and the number of patients will double by 2030. This, however, may be half of the real cases, because many people with DM have not been diagnosed. The rapid growth of DM associated with the growth of the elderly, obesity, unhealthy diet, and inactivity. Thus, treatments and lifestyle changes are prescribed and recommended [4].
Insulin enhances glucose uptake in muscle and fat and prevents its production in the liver. The shortage of insulin action or insulin resistance (IR) causes a significant imbalance in the uptake of glucose and leads to increased fasting and postprandial glucose levels [4-6]. Under normal conditions, the entrance of glucose in the blood leads to the rapid secretion of insulin, which is necessary for glucose entry into the cells through the cell membrane glucose-mediated transport proteins. A few seconds after insulin binding to the receptors, about 80% of membranes, especially of the muscle and fat cells become highly permeable to glucose. Various membrane-bound glucose transferring proteins, called glucose transporters (GLUT), among which GLUT1-4 are the most important facilitate glucose uptake into the cells [7-9]. Hyperglycemia, hyper/hypolipidemia, and hyperaminoacidemia which occur due to reduced insulin secretion or IR are indicators of DM and accompanied by the progressive trend of chronic DM complications [10-13].
Despite the great steps taken in treating and managing DM, we are witnessing a constant upward trend in DM complications and mortality rate [14]. However, due to the traditional anti-diabetic drug limitation, medical science is searching for options with fewer side effects and higher efficiency. The herbal medicines due to their ameliorating effects on diseases, fewer side effects, and rational prices have been used in all cultures as natural resources from ancient times. In DM it can be an alternative option that can remove the symptoms and prevent its complications. Also, there are some drugs, which can regenerate pancreatic β-cells, relieve IR, increase antioxidant activity, and reduce the cholesterol level [11-13]. It may be possible to manage the patients with type 2 diabetes via herbs and plants that decrease the blood glucose levels and at the same time increase glycogen storage in the liver, while there is no drug among the modern medicine with this property [11-13]. Today, the hypoglycemic effect of some plants has been proven in animal and human type 2 DM models. Moreover, metformin today recognized as the first-class drug for type 2 DM treatment, belongs to biguanide and produced from a plant. Other herbal medicines have been successful in treating DM; this subject itself justifies the efforts to test every one of the plants [3, 4].
One of the plants used to control DM is Prosopis farcta (P. farcta, scientific name) is known as Kahoorak plant (Persian name) and Syrian mesquite in English. It is a hypoglycemic drug which is used in traditional medicine to treat the patients with DM [9]. The plant belongs to the Leguminosae family and Mimosoideae subfamily which is a native and grown in an arid and semi-arid area in America, Africa, and Asia; in the western part of India and from China to Iran and Iraq. P. farcta contains secondary metabolite and effective compounds such as alkaloids; 5 hydroxyl L-arabinose, lektine, apigenin, and tryptamine. Also, the ethanol extract of this plant contains alkaloid, tannin, glycoside, flavonoid, and saponin. In Iran and some other countries, leaves of P. farcta are used in treating diarrhea, coughs, and colds [9]. Recently, the neuroprotective and general therapeutic effects on diabetic foot ulcers have been proven [15]. In Jordan, the extract of the roots is used to treat DM. The other therapeutic effects impact on stomach ulcers, rheumatism, laryngitis, angina, and dyspnea. P. farcta also has anti-spasmodic, anti-inflammatory effects and pain relief properties [15, 16].
3T3-L1 is a kind of cell line isolated from the primary fibroblastic embryonic cells of the mouse and used in research on fat tissues. Morphology of 3T3-L1 cells is similar to fibroblast in appropriate conditions; the cells are then differentiated into adipocyte-like phenotype. In fact, by increasing the synthesis and accumulating the triglycerides, they become similar to a signet ring. These differentiated cells are also sensitive to lipogenic and lipolytic hormones such as insulin [17, 18].
The present study aimed to test the effect of the aqueous and hydroalcoholic fruit extract of P. farcta on stimulating glucose uptake in 3T3-L1 adipocyte culture which has not been addressed before.
MATERIALS AND METHODS
DMEM high and low glucose medium for general growth and exposure differentiation respectively, purchased from Gibco and culture plates, insulin, glucose, glucose uptake measurement kit, Cytochalasin D supplied from Santa Crus, CA, USA.
Extract preparation:
The fruits were collected from a region around Semnan city and confirmed by the experts at the Agricultural Research Center in Semnan province. It was dried in a proper condition considering the light, temperature, and humidity of the chamber. To prepare the hydro-alcoholic extract with ethanol/methanol/water solvent (60, 30, 10), the Soxhlet instrument was used. P. farcta fruit (50 g) was solved into 500 mL of solvent and the hydroalcoholic extract was prepared by heating at 30-40 ° C for at least 4 times. Then the rest of the water was concentrated by rotary evaporator and the dried powder was collected and kept in the proper temperature for testing. Also, to prepare the aqueous extract of the fruit of P. farcta, the distiller in water was used. P. farcta fruits (100 g) were dissolved in 1000 mL of distilled water. Then, it was heated at 40 to 50 °C for two hours before filtered. The filtrated solution was dried using the oven completely. Powder of aqueous and hydroalcoholic extracts prepared via the extract process was used at concentrations of 20, 40, 80, 160 µg/mL, and the aqueous extract at the concentrations of 400 and 800 µg/mL were exposed to the cultured cells.
Cell preparation and cell culture:
In this study, 3T3-L1adipocyte cells line which has been purchased from the Pasteur Institute (Iran) were used in various groups with culture medium (DMEM) along with 10% FBS and the appropriate antibiotics under the equal and standard laboratory conditions and the amount of up-taken glucose was measured and calculated by glucose uptake measurement kit
The assessment 2-deoxy-glucose uptake
The 2 - Deoxy-glucose uptake by cells was measured using the glucose measurement kit according to manufacturer instruction. The 3T3-L1 adipocyte culture, 1500 cells/well in 100 μL of cell DMEM culture medium (FBS 10%, in 96- well plate) were incubated at 37ᵒC and 5% CO2 and differentiated cells were obtained after five days by using 2% FBS. The cells were incubated with 100 μL Kreps Ringer Phosphate Hepes (KRPH / 2% BSA) for 40 minutes to make severe glucose starvation after washed with a phosphate-buffered solution (PBS). Control group cells were washed three times with PBS and were kept until cell lysis step. Cells from the treated group were exposed to10 nM insulin for 20 min to activate GLUT proteins, 1 µM Cytochalasin D for 30 min (as an actin filament arrangement inhibitor), and the herbal extracts in a dose-dependent manner for 30 minute. In general, six treatment groups with glucose uptake stimulation were chosen: 4 with the hydroalcoholic extract of P. farcta at concentrations of 20, 40, 80, 160 µg/mL, and 2 with aqueous extract at 400 and 800 µg/mL respectively. 2-Deoxy Glucose, 2-DG, (10 mL of 10 mM solution) solution was added to cells and incubated for 20 min after mixing. Then 80 μL extraction buffer was added to each group of cells and the cell lysed solution is heated at a temperature of 85°C for 40 minutes that both the intracellular Nicotinamide Adenine Dinucleotide Phosphate (NADPs) are removed from cells and the enzymes existing in the sample will be destroyed. Then freezing the cell lysates on ice for 5 minutes and neutralizing them with 10 μL neutralization buffer before transfer supernatant to new tubes. The reaction is conducted in a flat bottom -96- well microplate and it is recommended to dilute samples 1/10 in assay buffer to a total volume of 50 μL. According to the kit instructions; 10 μL of Reaction Mix A (NADPH generation) is added to each well and incubated for one hour at 37 °C. Then 90 μL of extraction buffer is added to each well and microplate is sealed with parafilm and is heated for 40 minutes at 85°C. To degrade any NADP left in the sample, Microplate is incubated on ice for 5 minutes to be cooled and the reaction is neutralized by adding 12 μL of neutralizing buffer. Then according to the instructions kit, 38 μL of Reaction Mix B is added to each well and, they are mixed well by pipetting. Absorbing the color complex which has been produced in the wells with a wavelength of 412 nm is recorded using the ELISA microplate reader. Then, received data are analyzed based on the kit instructions.
Data analysis:
To describe numerical variables statistically, mean and standard deviation (M±SD) were used. The number and percentage of categorical variables reported. Data were analyzed via Chi- Square-2 Test and One-way ANOVA. In all tests, the reliable level was considered 95% and the significant level was less than 0.05.
RESULTS
A. differentiation of the 3T3-L1 adipocyte cells:
Microscopic images related to 3T3-L1 adipocyte cells morphology with an inverted microscope (Nikon model) at magnifications with 40 times before and after cell differentiation are shown in Figure 1. The differentiation in 3T3-L1 adipocyte cells plays an important role in biological function which about five days after FBS reduction, the differentiated cells were tested for 2-deoxy glucose uptake.
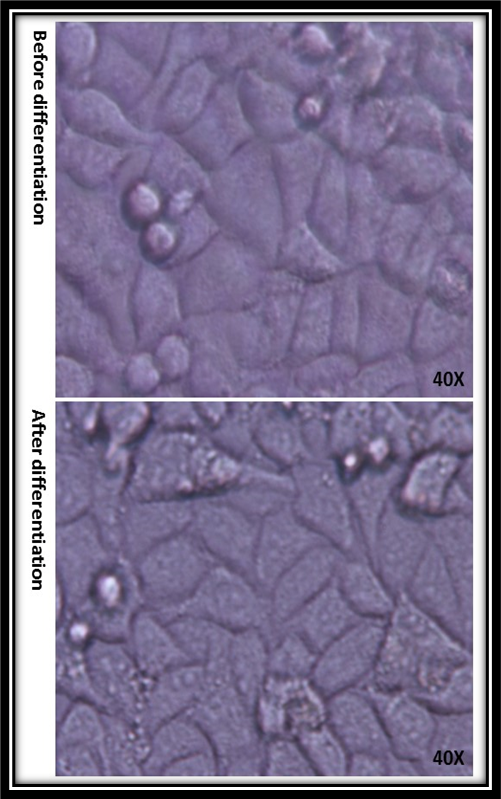
Figure 1: Nikon inverts microscopic images of 3T3 L1 cells before and after differentiation which is shown morphology when cells change after FBS reduction.
B. The effect of P. farcta fruit hydroalcoholic extract on glucose uptake in 3T3-L1 adipocyte cells
Glucose uptake was evaluated in 3T3-L1 adipocyte cells after exposed to P. Farcta fruit hydroalcoholic extract at doses of 20, 40, 80, and 160 µg/mL along with Cytochalasin D “as actin filaments depolymerization agent”. Insulin-stimulated glucose uptake increased significantly in comparison with the control group which is shown in Figure 2 which has been reduced with Cytochalasin D. Results showed that by increasing the concentration of P. farcta fruit hydroalcoholic extract glucose uptake in 3T3-L1 adipocyte cell line had increased significantly (p>0.001). Moreover, it was observed that glucose uptake level in 3T3-L1 adipocyte cells at the doses 80 and 160 µg/mL of P. farcta fruit hydroalcoholic extract was maximum and decreased significantly in presence of Cytochalasin D (p<0.001).
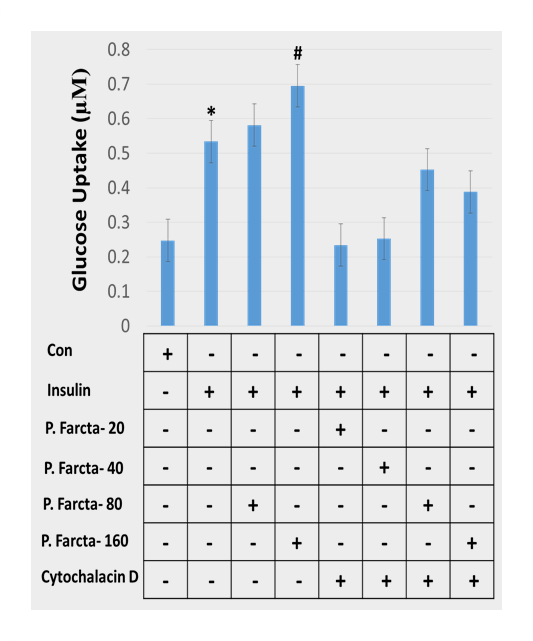
Figure 2. Dose-dependent impact of P. farcta fruit hydroalcoholic extract with Cytochalasin D on glucose uptake in the 3T3-L1 adipocyte cells. * P≤0.001.
C. The effect of P. farcta aqueous extract on Glucose uptake in 3T3-L1 Adipocyte cells
Figure 3 represented glucose uptake level in 3T3-L1adipocyte cells after exposed to P. farcta fruit aqueous extract at the doses of 400 and 800 µg/mL. Chi-square test showed a dose-dependent significant increase of glucose uptake in 3T3-L1 cell line (p>0.001) for both aqueous and hydroalcoholic extract of P. farcta with the greatest impact for 160 µg/mL of alcoholic extract and 800 µg/ml of the aqueous extract (Figure 4, 5).
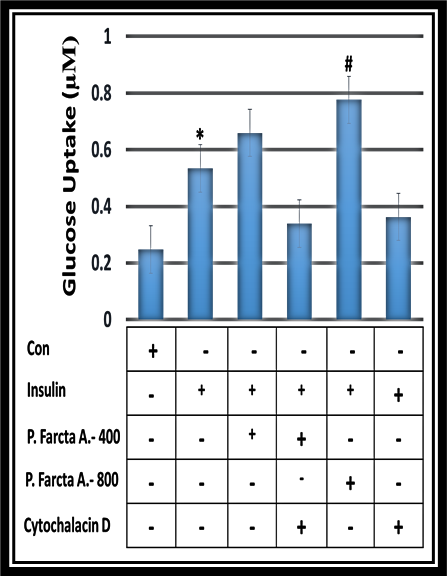
Figure 3. The effect of aqueous P. farcta extracts on glucose uptake level with insulin in 3T3-L1adipocyte cell lines (P≤0.001).
|
|
Figure 4. The effect of hydroalcoholic P. farcta fruit extract at doses of 80 and 160 and the aqueous P. farcta fruit extract at doses of 400 and 800 µg/mL on glucose uptake level in a 3T3L-1 adipocyte cell line (* P≤0.001).
Comparisons of the effect of hydroalcoholic P. farcta fruit extract at doses of 20, 40, 80, and 160 and aqueous P. farcta fruit extract at doses 400 and 800 µg/mL on glucose uptake level in 3T3-L1 adipocyte cells with Cytochalasin D are shown in figure 5. The results show that in the presence of any stimulator “as a control group” the cells have no reaction but in the presence of insulin, there is a significant increase in glucose uptake. Data analyzed by T-Test shows glucose uptake level in 3T3L1 adipocyte cell line in the hydroalcoholic and aqueous extract at doses of 160 and 800 µg/mL are more than treated cells with a lower dose (p˂0.001). Therefore, aqueous or alcoholic P. farcta fruit extract significantly in a dose-dependent manner stimulated glucose uptake in 3T3-L1 adipocyte cells which are significantly attenuated in presence of Cytocalasin D. (P<0.001) (Figure 5).
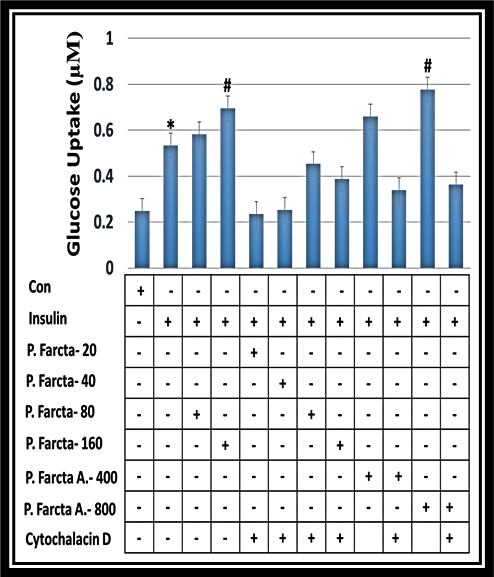
Figure 5. A comparison of hydroalcoholic P. farcta extract at doses of 20, 40, 80, and 160 µg/mL and aqueous P. farcta extract with doses of 400 and 800 µg/mL on glucose uptake in 3T3-L1adipocyte cells with Cytochalasin D. (* P≤0.001).
DISCUSSION:
The dramatic increase in metabolic syndromes, obesity, IR, and lipid disorders have led to the consideration of DM as a pandemic. Today, there are a lot of anti-diabetic drugs, including sulfonylureas, meglitinides, biguanides, alfa-glucosidases inhibitors and incretin-mimetics, etc [19]. A prospective study on diabetes in the UK (UKPDS) has shown that adequate glycemic control can prevent the vast majority of chronic DM complication [20]. Unfortunately, the current drug regimens are not able to establish a sufficient glycemic control in diabetic patients [21]. Herbal medicines are not only outdated but also play an important role in human health care. It is believed that about 1200 types of plants are effective in treating DM but only 400 types of them have been scientifically evaluated [21].
Therefore, this study examines anti-diabetic effects, one of the herbal medicines variant called P. farcta. To our knowledge is the first study which is evaluating the effect of aqueous and alcoholic P. farcta fruit extract on glucose uptake in 3T3-L1adipocyte cells using cell culture method. In this study, 3T3-L1 morphology before and after cell differentiation is investigated, that according to the conducted evaluation, there is a significant level of differentiation in 3T3-L1 adipocyte cells after FBS reduction. Moreover, data analysis is based on measuring the amount of 2-deoxy glucose using Abcam kit instruction that enters the cells. It was shown a dose-dependent significant increase of glucose uptake in the 3T3-L1 cell line (p>0.001) for both aqueous and hydroalcoholic extract of P. farcta with the greatest impact for 160 µg/mL of alcoholic extract and 800 µg/ml of the aqueous extract. On the other hand, we found a significantly higher glucose uptake level in 3T3-L1adipocyte cells with doses of 160 µg/mL of hydro/alcoholic extract and 800 µg/mL of aqueous extract as compared to all lower doses groups.
Recent studies, with different methods, have been conducted to assess the effect of this plant on DM. In agreement with our data, Dashtban et al. found that P. farcta beans extract significantly reduced the blood glucose level in the streptozotocin-induced diabetic rats [22]. Jarald et al. suggested that P. farcta is one of the several plants used in treating diabetes in Jordan and parts of Asia, as well as in some other places in the world and is used as an anti-diabetes drug [23].
On the other hand, Emad et al. in rats and rabbits with alloxan-induced DM, has not shown hypoglycemic effects of aqueous extract P. farcta. However, this study has several limitations. First, for all, the researchers did not determine the formulation of extract and the extracted organ. Sometimes, the whole plant extract has no significant effect on the treatment of certain factors, while the extract of different parts of the plant can be more effective [9].
Minaianet al. showed that the root extract of P. farcta was not effective in lowering blood sugar. According to the type of P. farcta organ to reduce blood sugar and contradictory studies, it seems that the fruit has a hypoglycemic effect [9]. Besides the direct anti-diabetic action, P. farcta it has some other properties such as anti-inflammatory effect, enhance of vascularization, and epithelialization that may be beneficial in diabetic ulcers wound healing. To confirm this Khayatzadeh and Ranjbar examined the effect of fruit pod powder and aquatic extract of P. farcta on cutaneous wounds in diabetic rats. Studies concluded that P. farcta has a hypoglycemic impact, vascularization, and anti-inflammatory properties and is also effective in wound healing of STZ-induced diabetic rats [15, 24, 25]. Various studies have reported the effect of aqueous and alcoholic extracts of P. farcta on glucose uptake and consequently DM treatment due to the antioxidant property of this plant. Obesity, DM, and other metabolic disease characterized by an imbalance between the body's antioxidant defense system and the production of free radicals and the number of free radical’s increases [19]. These oxidant factors cause lipid peroxidation and malondialdehyde (MDA) production. MDA is one of the important indicators to determine the amount of lipid peroxidation. P. farcta reduces the amount of MDA which is confirmed by the Hajinezhad study. The results showed that DM induction causes the liver and serum MDA increases; while P. farcta extract reduces MDA; histopathological findings also confirm the effect of this extract on healing diabetic lesions. Also, the results indicate hydro alcoholic P. farcta leaf extract reduces MDA concentration in serum and liver and prevents liver damage caused by DM. Some of P. farcta compounds are 5-hydroxyL-arabinose, lectin, and apigenin, and tryptamine. Also, the ethanol extract of this plant contains alkaloids, tannins, glycosides, flavonoids, and saponins [16].
The present study is one of the first studies to evaluate the effect of aqueous and alcoholic P. farcta fruit extract on glucose uptake in 3T3-L1adipocyte cells using cell culture method. So we can say this study has been able to investigate the anti-diabetic properties of this plant by evaluating the hydroalcoholic and aqueous P. farcta fruit extract on glucose uptake with different doses (hydroalcoholic extract at doses of 20, 40, 80 and 160 µg/mL and the aqueous extract at doses of 400 and 800 µg/mL) as a new subject with cell culture method. However, it is suggested that different doses of the other parts of P. farcta in vivo should be evaluated beside of and more accurate inspections on the effective compounds existing in this plant.
CONCLUSION:
The recent results showed a dose-dependent significant increase of glucose uptake in the 3T3-L1 cell line for both aqueous and hydroalcoholic extract of P. farcta with the greatest impact for 160 µg/mL of alcoholic extract and 800 µg/ml of the aqueous extract. Between the aqueous and hydroalcoholic P. farcta fruit extract, the dose of 800 µg/mL of aqueous has the most pronounced effect. Also, the glucose uptake in 3T3-L1adipocyte cells after doses of 160 µg/mL of hydroalcoholic and 800 µg/mL of aqueous P. farcta extract was significantly higher as compared to low doses treated groups. Thus, increasing 2-deoxy-glucose uptake in the hydroalcoholic and aqueous extract occurs in a dose-dependent manner. Based on current and further studies regarding P. farcta effective materials and hypoglycemic effect, it is expected that we would be able to use this plant as an adjunct to standard anti-diabetic therapy and for management of chronic DM complications. There is also a need for more accurate and clinical studies.
REFERENCES