



|
Study of Wound-Healing Ointment Composition based on Highly Dispersed Zinc Oxide Modified with Nanoscale Silver |
|
A.A. Blinova1, A.V. Blinov1*, O.A. Baklanova1, M.A. Yasnaya1, I.S. Baklanov1, A.A. Siddiqui2, A.B. Golik1, A.I. Okolelova3, A.A. Gvozdenko1, A.N. Simonov4, S.N. Povetkin1 |
|
|
|
1 North Caucasus Federal University, Pushkina Street 1, 355009, Stavropol, Russia. 2 Georgian National University SEU, Faculty of Medicine, 9 Tsinandali Street, Tbilisi, Georgia. 3 Kuban State Agrarian University named after I. T. Trubilin, Kalinina Street 13, 350044, Krasnodar, Russia. 4 Stavropol State Agrarian University, Zootekhnichesky Lane 12, 355017, Stavropol, Russia. |
ABSTRACT
A complex modern study of the ratio effect of components of an ointment composition based on highly dispersed Zinc oxide modified with nanoscale silver on its physical and chemical properties is performed. It was found that at a concentration of glycerol equal to C = 10%, and at a concentration of methylcellulose C = 3%, the value of the active acidity of the ointment composition is optimal (pH = 6.5-7). Studies of the structural and mechanical properties of the ointment composition have shown that the wound-healing ointment composition is elastic and has thixotropy, which makes it possible to use it in the treatment of wound surfaces with complex relief.
Key Words: Zinc oxide nanoparticles, Silver nanoparticles, Ointment composition, Physical and chemical properties, Structural and mechanical properties.
Ointments are soft medicines intended for application to skin areas, wound surfaces, or mucous membranes [1, 2]. In modern medicine, ointments are used in various fields of surgery, ophthalmology, gynecology, combustiology, etc. [3-9] The packaging of these ointments is also done in solid packaging with unreactive coating promoting the use of ointment compositions as promising for cosmetic purposes, for example, to soften the skin, nourish it with vitamins, trace elements, and treat various cosmetic skin imperfections e.g. freckles, removing age spots, hair, and others [10-14].
Ointments are the optimal dosage form, which consists of a combination of components of different chemical nature, aggregate state, and biological activity [15-18]. However, there are some disadvantages of using them, which include a limited range of pharmacological activity (unidirectional therapeutic effect) and irritating effect on treated skin [19-21]. To eliminate or minimize these shortcomings, it is significant to develop formulations of ointment compositions, optimize their composition, and study their properties.
The implementation of this work is related to our development - an ointment composition - which is a combined agent with antimicrobial properties (due to the content of nanosilver) and regenerative properties (due to the content of highly dispersed Zinc oxide).
Zinc oxide, being a source of the essential trace element Zinc, locally affects the skin area, releasing Zinc ions that saturate damaged tissues and promote accelerated regeneration of damaged skin [11, 22-29]. The ointment composition well moisturizes the damaged skin with the simultaneous restoration of the epidermis. This is an important aspect, especially for thermal burns. Due to the enhanced antimicrobial effect, silver nanoparticles stabilized with quaternary ammonium compounds. This prevents microbial contamination of the wound surface and inhibits secondary infection, which is an undoubted advantage of the ointment composition. Besides, nanoscale silver has a universal action and the absence of resistance to it by microorganisms [30-35].
The purpose of this work was to develop and optimize the technology for obtaining an ointment composition based on colloidal Zinc oxide modified with colloidal silver and to study its properties.
MATERIALS AND METHODS
The composition of the developed ointment includes the following components:
Nanoscale silver was obtained by chemical reduction in an aqueous medium. Silver nitrate (AgNO3) was used as a precursor and sodium borohydride (NaBH4) as a reducing agent. This allows the synthesis to be carried out at room temperature [36]. It provides the formation of a large number of small silver particles. The Quaternary Ammonium Compound (QAC) – Didecyldimethylammonium Bromide (DDAB) was used as a stabilizer for nanosilver particles. Stabilization of the particles is essential because the nanosilver is an unstable colloid system. Stabilization of silver nanoparticles by QAC can enhance the antimicrobial effect of nanosilver due to the synergistic effects on the microbial cells. That synergetic effect reduces the working concentration of nanosilver, which consequently leads to a decrease in consumption in the finished healing ointment composition. The process of obtaining nanosilver preparation stabilized with Didecyldimethylammonium bromide and studies of its biological activity are presented by Barabanov et al. (2018) [37].
Highly dispersed Zinc oxide was obtained by the sol-gel method in an alcohol medium [38-40]. This technique is relatively simple and does not require complex technological equipment, and is safe because of the low toxicity of reagents. The step-by-step process of synthesis of highly dispersed Zinc oxide is presented in the work of Blinov et al. (2016) [41]. Zinc acetate (Zn(CH3COO)2·2H2O) was used as a Zinc-containing precursor. The choice of this compound is since its samples consisting of smaller ZnO particles are obtained and also the remains of acetic acid completely evaporate at low temperatures (125-225oC) when drying the gels compared to sulfate and nitrate anions, which decompose at much higher temperatures (700-900°C).
The ointment composition based on highly dispersed Zinc oxide modified with nanosilver was prepared as follows. The required amount of MC-100 methylcellulose was mixed with distilled water and left to swell for 30 to 60 minutes. Then, the necessary amount of highly dispersed Zinc oxide (ZnO), glycerol, and nanosilver was added to the formed methylcellulose gel with constant stirring, followed by mechanical action to prevent the processes of destabilization and aggregation of ZnO and Ag nanoparticles. The result is a hydrophilic ointment composition with the following in table 1 ratio of components in mass %:
Table 1. Components composition of a hydrophilic ointment
|
Component |
Content, mass % |
|
Colloidal Silver |
0,0001 – 0,1 |
|
Colloidal Zinc Oxide |
5 – 15 |
|
Glycerin |
5 – 15 |
|
Methylcellulose |
0,05 – 5 |
|
Water |
remains |
The resulting ointment composition is an odorless white or yellow ointment, homogeneous inconsistency, and having a neutral reaction of the medium.
One of the most promising and modern methods of studying the rheological and Physico-chemical properties of ointment compositions is acoustic and electroacoustic spectroscopy [42-44]. The method is based on the measurement of the attenuation spectrum of an ultrasonic wave in a dispersed system and the subsequent mathematical processing of experimental data. This allows you to evaluate the following parameters: the speed of propagation of ultrasonic waves in the medium, the particle size of the dispersed phase, viscosity, ζ-potential, active acidity, and electrical conductivity. It is important that the spectrometer DT 1202 ("DISPERSION TECHNOLOGY" Inc., USA) can measure all parameters in defined dynamics, which allows to determine the shelf life of ointment compositions and study the influence of various factors on the entire colloidal system of ointments. Another advantage of acoustic and electroacoustic spectroscopy is the ability to study complex colloidal systems containing dispersed phases of several types (emulsions or suspensions), which include most ointment compositions.
For the study on the DT 1202 spectrometer, a series of samples of methylcellulose of the brand MC-100 with concentrations of 0,1 %; 0,5 %; 1 %; 2 %; 3 %; 4 % were prepared. At first, there was measured the attenuation spectrum of the ultrasonic wave.
The measurement of the structural and mechanical parameters of the developed ointment composition was carried out on a rotary viscometer "Fungilab Expert" ("Fungilab S. A.", Spain), the action of which is based on the use of viscous friction that occurs in a layer of liquid flowing in the annular gap between the rotating and stationary cylinders [45]. Each of the analyzed samples (about 20 g) was placed in a measuring tank. The speed of rotation of the cylinder was initially increased sequentially from a minimum at which the measurements could eventually increase to the maximum. After reaching the maximum limit for this device, the values of the tangential stress were also consistently reduced. To study the structural and mechanical properties of the ointment composition, the flow curves of the samples were plotted in the coordinates "shear rate – shear stress". During the destruction of structured systems (rising curve) with the increasing rotation speed of the inner cylinder is in a liquefaction system (decrease in viscosity). This never comes to an end, as a proportion of ties are reversibly restored even at high speeds. The downward curve reflects the system's ability to recover with a gradual decrease in the shear rate. The area enclosed between the ascending and descending curve is called the hysteresis loop. The area of the hysteresis loop can be used to judge the mechanical stability of structured systems: the smaller it is, the more mechanically stable the system is [28, 45].
RESULTS AND DISCUSSION
Physico-chemical Properties of Wound-healing Ointment Composition
At the first stage, the properties of the ointment base of the developed ointment composition were investigated. Since a properly selected base contributes to the maximum release of medicinal substances, therefore it then provides the best therapeutic effect. The choice of an ointment base should be justified by the set of properties that it should have:
In addition to the general conditions, many additional requirements are imposed on the ointment bases:
Methylcellulose is a simple ester of cellulose and methyl alcohol and is quite often used as a hydrophilic basis for ointment compositions [46]. Such valuable properties of its solutions as resistance to the effects of microorganisms, lack of toxicity, long shelf life, lack of taste and smell, as well as high binding, wetting, emulsifying, adhesive ability, the formation of a high-strength transparent film when drying, make it indispensable in the development of ointments formulations.
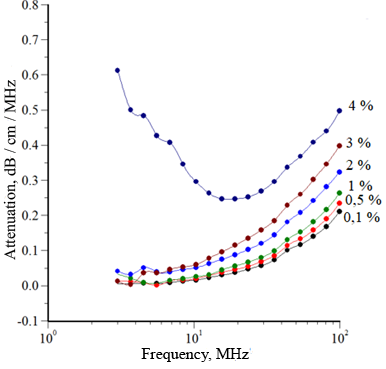
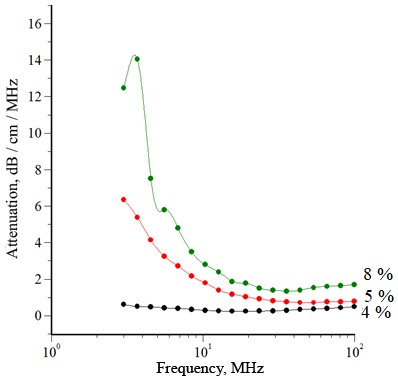
To study the properties of the ointment base, the ultrasonic wave attenuation spectrum was measured in samples of methylcellulose with different concentrations. The results are shown in Figure 1.
A
B
Figure 1. Attenuation Spectra of Ultrasonic Wave in Solutions of MC-100 with Various Concentrations:
A: 0,1 %; 0,5 %; 1 %; 2 %; 3 %; 4 %;
B: 4 %, 5 %, 8 %
Analysis of the data presented in Figure 1 showed that concentration of methylcellulose higher than 3% contributes to a sharp change in the spectral characteristics of MC-100 solutions, apparently caused by significant changes in rheological properties due to the formation of a spatial gel structure.
Figure 2 shows the dependence of the velocity of ultrasound propagation in the medium of methylcellulose solutions on its concentration.
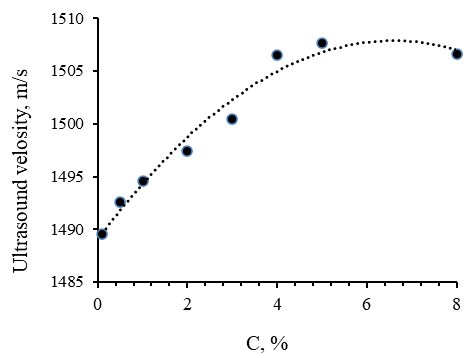
Figure 2. Dependence of Ultrasound Velocity on MC-100 Concentration Solutions
The graph shown in Figure 2 shows a significant change in the speed of ultrasound propagation at a concentration of methylcellulose greater than 3%. This confirms the formation of spatial gel structures at a concentration of MC-100 C ≥ 3%.
Figure 3 shows the dependence of active acidity of the pH of methylcellulose solutions on its concentration.
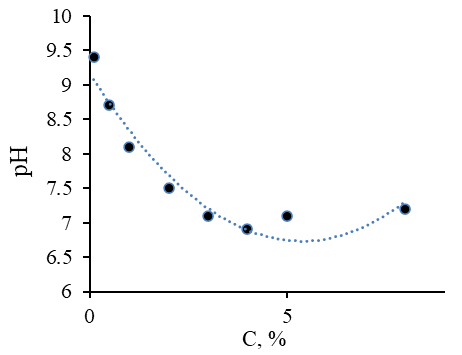
Figure 3. Dependence of Active Acidity on the Concentration of MC-100
Analysis of the Figure 3 shows a gradual uniform reduction of the active acidity in the range of 9.4 ≤ pH ≤ 7 at concentrations of methylcellulose from 0.1% to 4%,. With a further increase in the concentration of methylcellulose solutions to 8%, there is a uniform increase in the active acidity pH = 7,5. It, therefore, becomes important to note that the lowest concentration of MC-100 required to create an ointment composition with a neutral reaction of the medium is the concentration of methylcellulose of the order of 3 %.
The dependence of the Specific Electrical Conductivity (SEC) of aqueous solutions of methylcellulose on its concentration was also obtained. All measurements were also carried out on the DT 1202 spectrometer using built-in electrodes. The resulting relationship is shown in Figure 4.
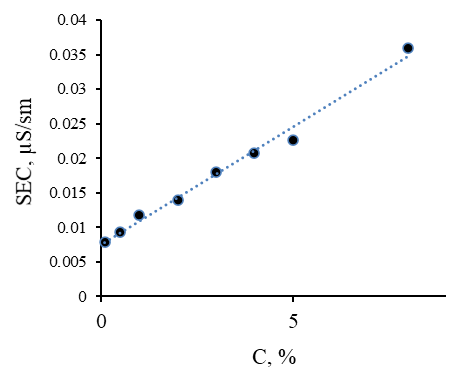
Figure 4. Dependence of Electrical Conductivity of Aqueous Solutions of Methylcellulose on the Concentration
The specific electrical conductivity of aqueous solutions of methylcellulose increases rectilinearly with increasing its concentration. The values of the specific electrical conductivity vary within 0.00779≤SEC≤0.0359 throughout the entire studied range of the concentration of methylcellulose. Small values of the specific electrical conductivity of methylcellulose solutions are associated with the absence of transitive components and weakly dissociating groups in methylcellulose.
The composition of the developed ointment composition includes such an auxiliary substance as glycerin, which is a moisture-retaining component. Due to the presence of glycerin, the ointment is evenly distributed over the treated surface. In addition, glycerin provides a gradual uniform drying of the ointment composition. Some properties of glycerol have been investigated. A series of solutions of glycerol in water was prepared, in which the dependences of the active acidity of the medium and the speed of the ultrasonic wave on the concentration of glycerol in the aqueous medium were obtained, shown in Figures 5 and 6, respectively.
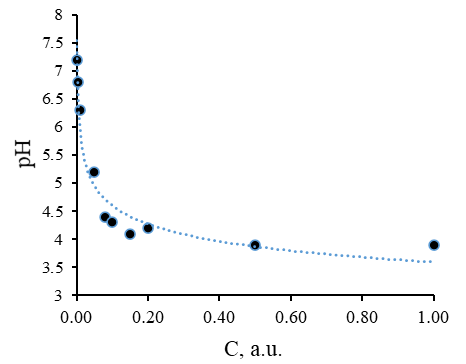
Figure 5. Dependence of Active Acidity of the Medium on the Concentration of Glycerol
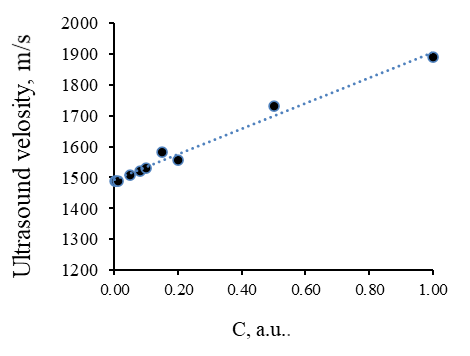
Figure 6. Dependence of Ultrasound Velocity in Aqueous Solutions of Glycerol
Dependency analysis presented in Figures 5 and 6 showed that the increase in glycerol concentration in aqueous solutions leads to a decrease in the active acidity of the medium. This is because glycerol is a weak acid. The appearance of protons in aqueous solutions is associated with the dissociation of the hydroxy groups within its molecule. The speed of ultrasound increases rectilinearly with an increase in the concentration of glycerol since aqueous solutions of glycerol are true solutions, and not colloidal. This specifies that the dissolution of glycerol in an aqueous medium is not accompanied by the formation of such a dispersed system as an emulsion.
Next, a series of samples of aqueous solutions of methylcellulose with different glycerol content was prepared. The concentration of methylcellulose was constant and was 3%, and the content of glycerol varied from 0 to 20 % (from 0 to 0.2 a.u.). In the obtained samples, the dependence of the active acidity of the medium on the content of glycerol in the mixture was determined (shown in Figure 7)
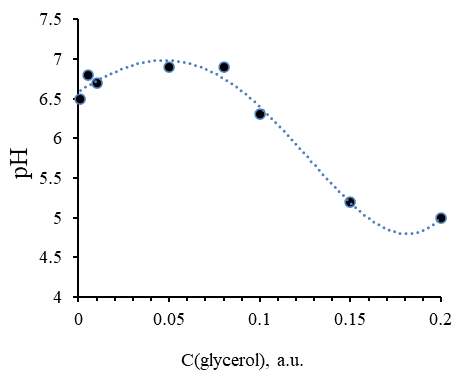
Figure 7. Dependence of Active Acidity of the Medium of 3% Aqueous Solution of Methylcellulose on Glycerol Concentration
The result of this experiment showed that the maximum possible concentration of glycerol was established, which was C = 10 % (0.1 a.u.), at which the values of the active acidity of the mixture are optimal (pH= 6.5–7). These properties characterize the high moisture-retaining ability of the ointment composition with an almost neutral reaction of the medium.
The results of the study show the prospects of using acoustic and electroacoustic spectroscopy to study various properties of ointment compositions.
Structural and Mechanical Properties of Wound Healing Ointment Composition
The structural and mechanical parameters of colloidal dispersions of methylcellulose in water (0,5 %, 1 %, 2 %, 3 %, and 4%) were measured. The resulting dependencies are shown in Figure 8.
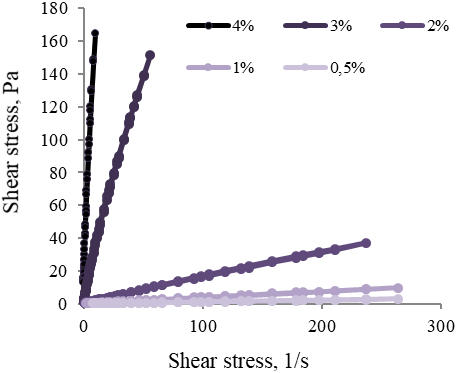
Figure 8. Rheograms Aqueous Solutions Flow of Methylcellulose
The obtained curves (Figure 8) form hysteresis loops with a minimum area, close to straight lines originating from the origin. This indicates a significant mechanical strength of gel-like structures formed by methylcellulose in an aqueous medium in the studied concentration range from 0.5 to 4% and poor thixotropic properties.
At a later stage, a series of methylcellulose solution samples with different glycerol content was obtained, in which the rheology of colloidal dispersions and their properties was studied. The content of methylcellulose in the system by weight was 3 %, the content of glycerol was 0.1; 0.5; 1; 5 and 8%. To study the rheological properties, the flow curves of the samples were plotted in the coordinates "shear rate – shear stress", shown in Figure 9.
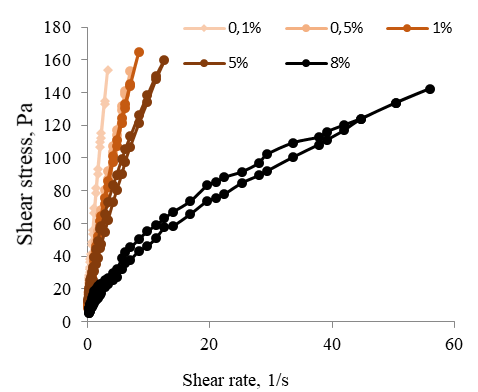
Figure 9. Rheograms of the Flow of 3 % Aqueous Solutions of Methylcellulose with Different Glycerol Content
The obtained curves at a glycerol concentration of 0.1, 0.5, and 2 % (Figure 9) show minor hysteresis loops approximating a straight line coming from the origin. The flow curves obtained from samples with glycerol concentrations of 5 and 8 % show significant hysteresis loops. In this case, the "ascending" curve characterizing the destruction of the system differs from the "descending" curve characterizing the restoration of the system. This is explained by the preservation of the residual deformation which takes place after a strong weakening of the structure under the influence of previously applied stress. The presence of ascending and descending curves of the hysteresis loop indicates that the samples under study have rheopexic properties. At low shear rates, the structure of the system is destroyed and completely restored (in this case, the system has the highest viscosity). With an increase in the shear rate, the destruction of the structure of the substance begins to prevail over the restoration, and the viscosity decreases. At high shear rates, the structure is destroyed, and the system begins to flow.
Further research was related to the measurement of the rheology of colloidal dispersion based on ZnO, glycerol, and methylcellulose in water. Colloidal dispersions based on glycerol in methylcellulose and water of various concentrations were studied. The content of glycerol in the system was 10 %, methylcellulose – 3 %, and Zinc oxide: 1, 5, 10, 15, and 20 %. To study the rheological properties, the flow curves of the samples were plotted in the coordinates "shear rate – shear stress" (Figure 10).
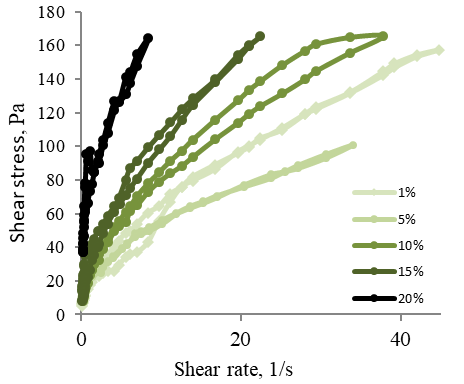
Figure 10. Rheograms of the Flow of 3% Solutions of Methylcellulose Containing 10 % Glycerol with a Different Content of Zinc Oxide
The obtained curves at the concentration of Zinc oxide 1, 5, 15, and 20 % (Figure 10) show minor hysteresis loops approximating a straight line coming from the origin. The curve obtained in the study of a sample with a concentration of Zinc oxide 10% is represented by a significant hysteresis loop. In this case, the "ascending" curve characterizing the destruction of the system differs from the "descending" curve characterizing the restoration of the system. This is explained by the preservation of the residual deformation after a strong weakening of the structure under the influence of previously applied stress. The presence of ascending and descending curves of the hysteresis loop indicates that the sample under study has rheopexic properties. At low shear rates, the structure of the system is destroyed and completely restored (in this case, the system has the highest viscosity). With an increase in the shear rate, the destruction of the structure of the substance begins to prevail over the restoration, and the viscosity decreases. At high shear rates, the structure is destroyed, and the system begins to flow.
CONCLUSION
The study of the influence of the component ratios (nanosilver, methylcellulose, glycerin) on the physical and chemical properties of developed ointment composition was successfully carried out. It was established that the maximum concentration of glycerol 10% and a concentration of methylcellulose 3% promote the optimal active acidity (pH=6.5-7). It characterizes the high moisture-retaining ability of the ointment composition with a slightly acidic and almost neutral reaction of the medium.
As a result of the study of structural and mechanical properties of the ointment composition, it was found that the wound-healing ointment composition based on highly dispersed Zinc oxide modified with nanosilver is elastic (makes it possible to use it for wound surfaces with complex relief) and has thixotropy (the ability to reduce the viscosity from mechanical action and increase the viscosity at rest).
References