|
Key Words: Seminal fluid, In vitro fertilization, Semen, Fungi, and Infertility
INTRODUCTION
Infertility is a disease of the reproductive system. It can be defined as the inability to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse. In vitro fertilization (IVF) is a process of fertilization where an egg is combined with sperm outside the body, in a laboratory dish [1]. Microbial assessment of seminal fluid in the weeks preceding in vitro fertilization (IVF) procedure is requested to avoid low sperm quality. Contaminated semen, by microorganisms, is the most frequent diagnosis of unsuccessful IVF. The quantity of the pathogenic fungi in semen has been reported to be a serious medical threat as the low-quality sperm cannot fertilize eggs in a laboratory tube. Generally, the deficiency of seminal fluid accounts for 7% of Human infertility [2]. Although fungi isolated from this fluid account for a small proportion of the total microorganism number, they pose opportunistic behaviors that affect sperm quality, along with distortions (especially aberrant motility) loss of DNA integrity, and deficient mitochondrial function [3, 4]. The most frequent pathogenic fungi in the seminal fluid were Candida spp. [5]. Personal habits such as sexual promiscuity and poor hygiene, increase the fungi isolates number and change the basic characteristics of semen.
In a hospital, the laboratory protocol uses several antibiotics to treat fungal pathogenic flora existing in seminal fluid to increase sperm quality. The fungal antibiotics used are amphotericin B, fluconazole, nystatin, flucytosine, econazole, ketoconazole, and miconazole [6].
The current study aims to isolate resistant fungi from seminal fluid and study their sensitivity to the antibiotics. It is hoped, by comparing the results obtained from client data, sperm quality will be improved along with increased rates of pregnancy using IVF at fertility clinics.
After obtaining ethical approval from the Biomedical Ethics Unit at King Abdulaziz University Hospital, we collected semen samples from sixty male subjects. The subjects were attending the in vitro fertilization clinics of King Abdulaziz University Hospital from October 2018 to October 2019. Proper hygiene was ensured in the collection of the samples which was done in one of the private rooms in the laboratory.
METHODS
Study population
After obtaining ethical approval from the Biomedical Ethics Unit at King Abdulaziz University Hospital, we collected semen samples from sixty male subjects. The subjects were attending the in vitro fertilization clinics of King Abdulaziz University Hospital from October 2018 to October 2019. Proper hygiene was ensured in the collection of the samples which was done in one of the private rooms in the laboratory.
Specimen Collection
Semen specimens were collected by masturbation after 3–7 days of abstinence. Before the sample collection, instructions were provided to the participants on procedures to be followed to avoid sample contamination. The subject's hands were washed using soap more than two times. The penis, especially the glans and the coronal sulcus, was washed with warm soapy water and swabbed after with 75% alcohol more than two times. Without any contact with the interior of the sterile wall of the container, the semen was then ejaculated into a sterile glass receptacle. The freshly provided seminal fluid was used for routine semen clinical examination, Gram staining, microscopy, and molecular technique. Within 2 hours of collection, the remainder of the semen samples were transferred to sterile Eppendorf tubes and stored at 80°C. The exclusion criteria are men using antibiotics for less than three months before sampling and patients with chronic diseases such as prostate cancer and HIV. We will be informed about the time of the IVF procedure of each client.
Cultures
The following fungi culture media plates were used: Sabouraud dextrose agar (SDA) and yeast peptone dextrose agar (YPD). All seminal fluid samples were inoculated directly using 100 µl of the sample was spread by L-loops in triplicate on the previous media and incubated for 5 days at 25°C and 37°C. The growth was examined for size, shape, elevation, and other cultural characteristics. Lactophenolcotton blue cotton blue has stained the isolates and pure growth was subjected to sensitivity to a range of antibiotics testing according to standard [7].
Microbial analysis
The total number of colonies was done by manually counting of colonies on plates and the results were calculated as the mean of colony-forming units per milliliter (CFU/ml). The limit of detection was 103 CFU/ml as described by Pelzer [8].
Morphological structures analysis
All isolates were phenotypically identified from pure cultures. Colony characteristics including growth rate, colony color, texture, size, and shape were considered as important diagnostic criteria for identification and recorded as macro-morphological structures. The micro-morphological structure was observed in wet mounts prepared on microscopic slides and stained with LPCB using the standard protocol [9].
Molecular identification of microbial isolates
All samples were mixed with 70% glycerol in Eppendorf tubes and store in 80ºc. Then the samples were sent to Macrogen Company, Seoul, South Korea for purification and sequencing.
DNA Sequencing
Sequence identities were characterized using BLAST GenBank general databases from the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov) [10]. The sequence alignment of the constructions of neighbor analysis was done by theBLAST and MEGA-X program (version 10.0.5). The result reports were received from the Macrogen molecular analysis laboratory and the sequencing data were submitted to GenBank and the obtained accession number was recorded for each isolate.
Antimicrobial susceptibility test
The susceptibility to five antifungal agents was determined using the VITEK 2 antifungal susceptibility testing system (bioMerieux, Durham, USA) (card number AST-YS08) to determine the antifungal susceptibility of yeasts compared with the Clinical and Laboratory Standards Institute (CLSI M100-S25, 2015) reference standards within 24 hours. The antifungal used included Caspofungin (CAS) (0.25-4 ug/ml), Fluconazole (FLU) (1-64ug/ml), Flucytosine (FCT) (1-64ug/ml), Micafungin (MCF) (0.06-4 ug/ml), and Voriconazole (VRC) (012-8ug/ml).The cassettes were loaded and placed into the VITEK-2 instrument, and the suspensions were diluted by the instrument. Afterward, the cards were filled, incubated, and read automatically. The complete data for each fungal isolate were expressed as minimal inhibitory concentrations (MICs) in micrograms per milliliter (µg/ml).
Statistical Analyses
Data collected were analyzed using SPSS to determine the significant difference among the data (Armonk, NY: IBM Corp). A P-value of < 0.05 was considered statistically significant.
RESULTS
Microbial isolation
The result of microbial isolation of semen specimen was collected from the client would go for the IVF procedure. Overall, 17 (28%) of the 60 semen specimens were positive for fungal growth, while no fungal colonies were observed on 43 (72%) specimens. All fungal colonies were counting with counting forming unit (CFU) table (1). The fungal colonies grew on the two different media with two different temperatures 25°C and 37°C but with the different growth rates. The total CFU/ml of all fungal colonies isolated from different media at 25°C were 872.1×103 CFU/ml whereas 727.2×103 CFU/ml at 37°C. In the SDA medium, the total CFU/ml of fungal colonies grown at 25°C were 331.1×103 CFU/ml while the total CFU/ml grown at 37°C were 541×103 CFU/ml. Furthermore, the total CFU/ml of yeast colonies grown on YPD medium at 25°C were 64.2×103 CFU/ml, and 333.3×103 CFU/ml at 37°C.
The fungal morphological features were identified up to the genus level according to the morphological reference published [11]. The color of all the isolates is creamy to yellowish, and depending on the species, the colony has a glistening, smooth, or dry texture. Under microscopic analysis withLPCBstain,allfungalisolates showed yeast cells with blastoconidia produce singly or in small clusters as shown in Figures (1, 2, and 3) and followed by Candida guilliermondii (60%), Candida parapsilosis (23%), Candida glabrata (17%).
The effect of the microbial load was also considered in this study. The quantitative method estimated the diffusion of yeast within the semen samples. The yeast species were isolated at concentrations ranging from 102 CFU/ml to >103 CFU/ml in semen samples. There manifest to be no correlations between the microbial load in semen and the etiology of infertility
|
|

|
|
(a)
|
(b)
|
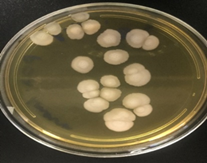
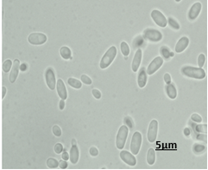
Fig. 1: The isolate of C. guilliermondii(a) coloniesrepresentsflat, moist, smooth, and cream to yellow on SDA.(b) Cellular morphology of yeast cells stained with LPCB and observed blastoconidia produce in clusters in size 5μm under 100x objective by light microscopy.
|
|
|
|
(a)
|
(b)
|
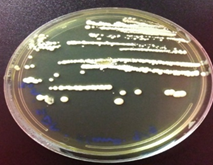
Fig. 2: The isolate of C. parapsilosis(a) coloniesrepresentswhite, creamy, and shiny colonies on SDA (b) Cellular morphology of yeast cells stained with LPCB and observed small blastoconidia produce singly in size 5μm under 100x objective by light microscopy.
 |
 |
|
(a)
|
(b)
|
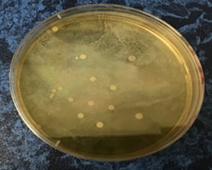
Fig. 3: The isolate of C. glabrata (a) coloniesrepresentswhite, smooth, and glistening colonies on SDA. (b) Cellular morphology of yeast cells stained with LPCB and observed small blastoconidia produce in clusters in size 20μm under 100x objective by light microscopy.
Molecular identification
Initially inoculated a single colony of culture from YPD agar into 5 ml of YPD broth and incubated for 48 h at room temperature in a shaking incubator (SI-100, Human lab, Gyeonggi-do, South Korea). The cells were harvested from 1 ml of the culture broth in an Eppendorf tube mixed with 70% glycerol then store at -80ºC. Then the samples were sent to Macrogen Company, Seoul, South Korea for DNA purification and sequencing.
The PCR genetic sequences alignment of yeast isolates was determined as known species based on the 99% similarity of their sequences with that of the known species already published in NCBI databases. All oftheyeastspecies isolates could be characterized byCandida with three different species as C. guilliermondii for SF1, C. glabrata for SF2, and C. parapsilosis SF3. New accession numbers were recorded of the mentioned fungal strains in the GenBank database as shown in the table. C. guilliermondii identified similarities percentages 99% for the SF1. SF2 was showed 99% similarity with the type strain C.parapsilosis and C. glabratareporteda similarity amount of 99% for the SF3as shown in Table (1).
Table 1: List of yeast strains GenBank accession number isolated from semen and % identity with closely related strains found in the NCBI website.
Antimicrobial test
According to CLSI documents, the candida isolates were indicated different antifungal sensitivity to five antifungal agents including CAS, FLU, FCT, MCF, and VRC unless C. para psilosis that not advanced any MIC values against fluconazole with an analysis message (this antibiotic is not claimed). Micafungin showed the highest susceptibility to isolates of C. parapsilosisat (≤ 0.06 µg/mL)and VRC showed the highest susceptibility to the other two isolates of C. glabrataand C. guilliermondiiat (≤ 0.12 µg/mL) as shown in Table (2).
Table 2: scale azole MICs (in mm) of antifungal agents for the Candida species isolated from seminal fluid.
|
Candida species
|
CAS
|
FLU
|
FCT
|
MCF
|
VRC
|
|
MIC
(µg/mL)
|
MIC
(µg/mL)
|
MIC
(µg/mL)
|
MIC
(µg/mL)
|
MIC
(µg/mL)
|
|
C. guilliermondii
|
0.5
|
2
|
≤ 1
|
0.5
|
≤ 0.12
|
|
(S)
|
(S)
|
(S)
|
(S)
|
(S)
|
|
C. parapsilosis
|
0.12
|
-
|
≤ 1
|
≤ 0.06
|
≤ 0.12
|
|
(S)
|
(-)
|
(S)
|
(S)
|
(S)
|
|
C.glabrata
|
0.25
|
2
|
≤ 1
|
0.5
|
≤ 0.12
|
|
(S)
|
(S)
|
(S)
|
(S)
|
(S)
|
Scale azole of the minimum inhibitory concentration (MICs) was measured using the VITEK-2 system. (S) Susceptible; (R) Resistant. CAS, caspofungin; FLU, fluconazole; FCT, flucytosine; MCF, micafungin; VRC, voriconazole; NS, MIC value not showed with analysis message (this antibiotic is not claimed).
DISCUSSION
In the present study, three Candida species were identified from 17semen specimens. These yeasts were present in 28% of semen while there were 72% of semen did not show any fungal growth. However, no complete agreement on the detrimental role of the presence of fungi in the semen [12]. The most common pathogenic fungi isolated from semen were Candida albicans and these fungal infections may adversely affect reproduction and may consequently influence ejaculate production [13].
Opportunistic microorganisms that can usually cause some classical infections of the urogenital tract as prostatitis and epididymitis and subclinical reproductive tract infections in many cases. Some possible pathophysiological mechanisms that could cause the development of infertility is associated with the presence of microorganisms in the ejaculation [14]. The seminal fluid may be contaminated by the hematogenous invasion of different microbes spread via the oral mucosa and skin. It is may also be colonizedwithcollecting semen samples procedure [15]. In our study, Candida spp.is a common commensally yeast of the male reproductive tract and investigated as opportunistic pathogens that may sometimes cause diseases and it canaffect fertility in several ways including damage of spermatozoa, hampering their motility. infection Mostly cases were diagnosed amount (80% to 90%) infected with Candida albicans, while infection with other species such as C. glabrata and C. parapsilosisoccurs less frequently [16]. The presence of C. Albicans, C.glabrataaffects seminal parameters including motility, viability, and sperm morphology [17].
On the other hand, several researchers reported yeast species based on a molecular technique to confirm species determination [10]. It is a big challenged to categorize between different yeasts because they have very comparable morphological structures. The molecular identification for the microbial strains exposed that the identification of yeast isolates using the morphological features was practically giving genus scientific name, thus submission of the correct identification for all yeast isolates tested were required to distinguish species name. Many studies have viewed the effectiveness of microbial semen contamination in male fertility; however, the putative detrimental effect of fungi on sperm quality is still controversial [18]. Fungi can impact on the male reproductive ability, causing the agglutination of motile sperm, decreasing the causing alterations in cell morphology and indirectly, ability of acrosome reaction, and through the production of reactive oxygen species generated by the inflammatory response to the infection [19].
The relationship between pathogenic fungal growth and male infertility, as well as some quantitative and qualitative features of the sperm, has been studied. Researchers noted that the most common fungal isolate was C. spp. Therefore, the presence of fungi in the seminal fluid may strongly affect the survival of sperm [20], these findings have confirmed in the present study. The role of infectious yeast such as Uropathogenic sp. and Candida tropicalis in the seminal fluid decreased sperm density, motility, and morphology, resulting in increased male infertility [21]. On the other hand, the presence of pathogenic fungi in seminal fluid failed to show the cause-and-effect relationship between fungi infections in seminal fluid and male infertility. To further complicate the problem, the presence of fungi in sperm samples of infertile men has a prevalence similar to that seen in fertile men. Therefore, the clinical significance of fungi in seminal fluid is still unclear [22].
Investigators have demonstrated that antibiotic treatments reduced the number of fungal isolates and increased ejaculate volume, spermatozoa, number of motile spermatozoa, sperm concentration, and normal morphology [13]. The Candida species have been detected in semen samples and have been shown to harm the IVF success. After antibiotic treatments, the reduction of Candida growth has been shown to correlate with increased IVF pregnancy rates. [23]. Maciejewska et al., (2005) supports our finding with antibiotic treatment for the genitourinary tract infection (GTI) resulting in ad a significant improvement in sperm volume, movement, sperm concentration, morphology, and vitality in multiple samples resulting in increased IVF success. Moving on from this research, Bielanski attempted a preventive protocol with an embryo culture media were mixed with fungal antibiotics to reduce pathogenic microorganisms' development [24].
The fresh semen may contain different kinds of fungi. Yeasts like Candida parapsilosisare skin commensals. we can reduce them by rigorous hygiene of the collection and processing rooms. The yeasts’ concentration in semen samples is quite constant indicating an internal source of contamination [25]. The literature concerning the fungal contamination of male semen is relatively poor and it could be a justification for the opportunity of our study. The embryo culture media is mixed with fungal antibiotics such as amphotericin B, fluconazole, nystatin, flucytosine, econazole, ketoconazole, and miconazole [6]. Also, all C. spp in this study were sensitive with five common antifungal agents, unless C.parapsilosisthat not advanced any MIC values against fluconazole.
The finding of this study showed that 28 % of semen culture was found to be fungal isolates. C. spp was found the commonest organism followed by C. guilliermondii, C. parapsilosis, and C. glabrata. The regular screening of fungal pathogens in infertile man seems necessary because it affects infertility in several ways.
CONCLUSION
This research investigated the fungal isolates within the seminal fluid of males before the IVF procedure. The semen was not always sterile but contained a diverse range species of Yeasts that may reduce the IVF outcomes. Furthermore, the Identification of fungus within the semen in males with repeated unsuccessful IVF cycles may extend a chance to initiate antifungal treatment before the next conception.
ACKNOWLEDGMENT
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah supported this project and, therefore, the authors acknowledge this and thank the DSR for technical and financial support.
REFERENCES
- Gosden, R. Jean Marian Purdy remembered–the hidden life of an IVF pioneer. Human Fertility, 2018; 21(2), 86-89.
- Lotti, F., Maggi, M. Ultrasound of the male genital tract in relation to male reproductive health. Human Reproduction Update, 2015; 21(1): 56-83.
- Berjis K, Ghiasi M, Sangy S. Study of seminal infection among an infertile male population in Qom, Iran, and its effect on sperm quality. Iran. J. Microbiol. 2018;10:111–116.
- Robertson, S. A., Sharkey, D. J. Seminal fluid and fertility in women. Fertility and Sterility, 2016; 106(3), 511-519.
- Bader, O., Weig, M., Taverne-Ghadwal, L., Lugert, R., Gross, U., Kuhns, M. Improved clinical laboratory identification of human pathogenic yeasts by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clinical Microbiology and Infection, 2011; 17(9): 1359-1365.
- Osoba, A. O., Al-Mowallad, A. W., McAlear, D. E., Hussein, B. A. Candidemia and the susceptibility pattern of Candida isolates in blood. Saudi medical journal, 2003; 24(10): 1060-1063.
- Angelis, G., Menchinelli, G., Torelli, R., De Carolis, E., Posteraro, P., Sanguinetti, M., Posteraro, B. Different detection capabilities by mycological media for Candida isolates from mono-or dual-species cultures. PloS one, 2020; 15(3), e0226467.
- Pelzer, E.S., Microbial colonization of human follicular fluid and adverse in vitro fertilization outcomes. Doctoral dissertation, Queensland University of Technology, 2011.
- Carter, G.R., Cole Jr, Diagnostic procedure in veterinary bacteriology and mycology. Fifth ed. 2012: Academic Press, Inc.
- Najjar, A., et al., Seasonal Variations of Fungi Isolated from Swimming Pools in Jeddah, Saudi Arabia.Middle-East Journal of Scientific Research 2019. 27(1): 55-63.
- Kidd S, Halliday C, Alexiou H, Ellis D. Descriptions of medical fungi. Third ed. 2016, Australia.
- Maciejewska, D., Ciupińska, M., &Kurpisz, M. Bacterial infection and semen quality. Journal of reproductive immunology, 2005; 67(1-2), 51-56.
- Skau, P. A., Folstad, I. Do bacterial infections cause reduced ejaculate quality? A meta-analysis of antibiotic treatment of male infertility. Behavioral Ecology, 2003; 14(1), 40-47.
- Bhatt, C. P. Bacterial pathogens in semen culture and their antibiotic susceptibility pattern in vitro. Int J Biomed Res 2015; 6 (11): 909-914.
- Król, J., Żarski, D., Bernáth, G., Palińska-Żarska, K., Krejszeff, S., Długoński, A., Horváth, Á. Effect of urine contamination on semen quality variables in Eurasian perch Percafluviatilis L. Animal reproduction science, 2018; 197, 240-246.
- Soong, D., Einarson, A. Vaginal yeast infections during pregnancy. Canadian family physician, 2009; 55(3): 255-256.
- Duque, E. X., Suárez, J. P., Maya, W. D. C. Yeast and fertility: Effects of in vitro activity of Candida spp. on sperm quality. Journal of reproduction & infertility, 2018; 19(1), 49.
- Borges, E. D., Berteli, T. S., Reis, T. F., Silva, A. S., Vireque, A. A. Microbial contamination in assisted reproductive technology: source, prevalence, and cost. Journal of assisted reproduction and genetics, 2020; 37(1), 53-61.
- Tremellen, K. Oxidative stress and male infertility—a clinical perspective. Human reproduction update, 2008; 14(3), 243-258.
- Ekhaise FO, Richard FR. Common bacterial isolates associated with semen of men attending the fertility clinic of the University of Benin teaching hospital (UBTH), Benin City, Nigeria. African Journal of Microbiology Research. 2011; 5 (22): 3805-3809.
- Sasikumar, S., Dakshayani, D., Franklin, A., Samuel, R. An in-vitro study of effectiveness of uropathogenic yeast on male infertility. International Journal of Current Microbiology and Applied Sciences, 2013; 2(5), 233-246.
- Ombelet, W., Bosmans, E., Janssen, M., Cox, A., Vlasselaer, J., Gyselaers, W., ... Steeno, O. Semen parameters in a fertile versus subfertile population: a need for change in the interpretation of semen testing. Human Reproduction (Oxford, England), 1997; 12(5), 987-993.
- Shalika, S., Dugan, K., Smith, R. D., Padilla, S. L. The effect of positive semen bacterial and Ureaplasma cultures on in-vitro fertilization success. Human reproduction, 1996; 11(12), 2789-2792.
- Bielanski, A. Disinfection procedures for controlling microorganisms in the semen and embryos of humans and farm animals. Theriogenology, 2007; 68(1), 1-22.
Cıorneı, Ş., Roşca, P., Drugociu, D. Bacterial and fungal burden in boar semen. Research Journal of Biotechnology, 2012; 7(4), 23-27.
|



