



|
Investigation of Antimicrobial properties of the Extract and Nano-extract of the Arial organ of Hypericum Perforatum
Sepideh Tabrizie¹, Elham Mahdian¹*, Ali Mohammadi Sani¹, Mahboobe Sarabi-Jamab², Fatemeh Oroojalian³ |
|
1Department of Food Science and Technology, Quchan Branch, Islamic Azad University, Quchan, Iran. 2Department of Food Biotechnology, Research Institute of Food Science and Technology (RIFST), Mashhad, Iran. 3 Department of Advanced Sciences and Technologies, North Khorasan University of Medical Sciences, Bojnurd, Iran. |
ABSTRACT
Background: Nowadays, it is necessary to discover new and efficient antifungal or antimicrobial compounds because of the increasing antibiotic-resistant microorganisms. The use of medicinal plants for the natural treatment of diseases of microbial origin has been mainly considered. Introduction: Hypericum Perforatum is a medicinal plant that has been mentioned in traditional medicine for its antimicrobial effects. In this study, the extract of Aerial organs of Hypericum Perforatum was prepared by hydroalcoholic solvent and the resulting extract was coated in nanoparticles of calcium alginate, and the antimicrobial properties of the extract and nanoparticles were investigated on four groups of gram-positive, gram-negative, yeast, and mold microorganisms. Materials and Methods: First, the hydroalcoholic extract and nanoparticles of Aerial organs of Hypericum Perforatum were prepared and their antimicrobial properties against Escherichia coli, Staphylococcus aureus, Candida albicans, and Aspergillus parasiticus were evaluated by disc diffusion method and determining the diameter of inhibition zone. Also determining the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal or Fungicidal Concentration (MBC/ MFC) were investigated. Results: The results of the disc diffusion method showed that the biggest inhibition zone was achieved for Candida albicans by using a nano-capsule containing the extract. In the presence of the extract, the diameter of the zone of inhibition created against Candida albicans, Staphylococcus aureus, Escherichia coli, and Aspergillus parasiticus was 32, 15, 12 and 0 mm. Also, the Nano-extract caused the inhibition zone diameter of 21, 18, 35, and 0 mm, respectively, for these microorganisms. The MIC and MBC of the extract against Escherichia coli, Staphylococcus aureus, Candida albicans, and Aspergillus parasiticus were 100, 50, 25, and >200 mg/ml, respectively, while the MIC and MBC of Nano capsule containing extract was 12.5, 6.25, 3.125 and >200 mg/ml. According to these results, the antimicrobial effect of nanocapsules containing extract was higher than the extract with the same concentration. Also, the results showed that the extract and nanocapsule containing extract, at the highest concentration, had not antifungal properties against Aspergillus parasiticus, whereas the lowest MIC and MFC in both extract and Nano-extract belonged to Candida albicans. Conclusion: Comparison of the effect of Hypericum Perforatum extract and Nano-particle containing extract on four groups of microorganisms showed that Nano-extract had more antimicrobial effect on microorganisms compared to the extract with the same concentration.
Keywords: Hypericum Perforatum, Hydroalcoholic Extract, Nanoemulsion Particle, Disk Diffusion, Microplate Dilution.
INTRODUCTION
In recent years, antibiotics are the main drugs in the treatment of microbial infections, but due to the emergence and spread of resistance of microorganisms to antibiotics
such as erythromycin, gentamycin, tetracycline, etc., efforts to find new antimicrobial sources has become very important. Therefore, the use of medicinal plants in the treatment of microbial infections has been demonstrated [1-3].
Among other factors that have increased the use of medicinal plants are the side effects of chemical drugs and, conversely, the lower cost of herbal medicines [1]. Numerous studies have shown the antimicrobial effects of many medicinal plants [4, 5].
Hypericum Perforatum is a herbaceous, perennial herb about one meter tall with a pleasant odor. Other names for Hypericum Perforatum include "Rai flower, Hezar Cheshmeh flower, and tea grass". The medicinal part of the plant is its aerial organs. Bright and dark spots can be seen on its leaves. There are bright spots on all parts of the leaf and it is the place where the essential oil accumulates [6-9].
Some of the important properties of this plant in traditional medicine are diuretic, antiseptic, analgesic, anti-gout, effective in the treatment of rheumatism and chronic gastrointestinal spasms, sciatica, and infectious diseases such as syphilis, tuberculosis, dysentery, whooping cough, worm excretion, and malaria treatment [6, 9].
Using various methods of extracting extracts such as massaging (wetting), percolation, beetles, etc., including different parts of the plant, extracts with different characteristics can be obtained [10-11].
In most studies on the antimicrobial properties of plant extracts, extraction has been done by conventional methods with solvent. Solvent extraction is a common method for extracting compounds from solids or liquids. This method is able to extract high-value compounds from various plant sources.
One of the ways to increase the effectiveness of extracts is to prepare Nano-emulsions. Nano-emulsion is used in the process of emulsion formation for the production of nanoparticles. Nanoparticles are very fine particles in the range of 1 to 100 nanometer with special physicochemical properties, higher surface to volume ratio, and suitable interaction power with surrounding biological systems. By converting natural bioactive compounds (such as extract containing antimicrobial agents) to nanoscales, their properties will be enhanced due to decreasing the size and increasing the surface; therefore, they could pass through the pores of the peptidoglycan cell wall and thus have a greater effect compared to the original bioactive compounds [12-15].
The aim of this study was to evaluate the efficacy of nanoparticles produced from the hydroalcoholic extract of the aerial organs of Hypericum Perforatum in comparison with the extract itself against the growth of Staphylococcus aureus, Escherichia coli, Candida albicans, and Aspergillus parasiticus.
MATERIALS AND METHODS:
Microorganisms and ingredients:
The four microorganisms included Staphylococcus aureus (ATCC 1431), Escherichia coli (ATCC 1399), Candida albicans (ATCC10231), and Aspergillus parasiticus (ATCC1709) were prepared from the microbial bank of the Scientific and Industrial Research Organization of Iran; Also, Muller Hinton Agar and broth (MHA/MHB) and other solvents and chemicals used in this study were supplied by Merck Company.
Extraction:
The plant’s aerial organs were prepared from a farm and completely dried in a place away from sunlight at a temperature of about 24 °C for a week. The sample was ground and extracted by water/ethanol solvent in a ratio of 30 to 70. After 48 hours, the extract was centrifuged at 3000 ×g for 10 minutes after passing through a cloth filter. Then, it was placed inside a rotary evaporator at 40°C for solvent evaporation. After that, the extract was placed in an oven at 40 °C to dry completely. The dried extract was kept at 4 °C until the experiments
Preparation of Nanoparticle containing extract
Due to its ability to enhance the antimicrobial effect, the nanoemulsion structure is a good choice for effectively inserting the extract into the cell. The emulsion method was used for the preparation of calcium alginate Nanocapsules containing the extract. The ratio of the extract (at a concentration of 100 mg/ml) to sodium alginate was 1: 4. The nanocapsule containing the extract was produced by adding calcium chloride nanoparticles to the alginate containing the extract in a ratio of 1: 6 and over a period of 4 hours [15]. The properties of calcium alginate nanocapsule caontaining the extract are shown in Table 1.
Table 1. Particle size, Particle size dispersion index, Zeta potential of Hypericum Perforatum Nanocapsule
|
Samples |
Particle size (nM) |
Particle size dispersion Index |
Zeta potential (EV) |
Efficiency of encapsulation (%) |
|
Nano Capsule of Hypericum Perforatum |
500 |
0.21 |
-38.3 |
85 |
Disc diffusion Method performance
To activate the lyophilized Staphylococcus aureus, Escherichia coli, and Candida albicans were transferred into Mueller-Hinton Broth medium and incubated at 37 °C for 18-24 hours. Then, the turbidity equivalent 0.5 McFarland standard was prepared and diluted to 106 CFU/ml. For Aspergillus parasiticus, the hemocytometer chamber was used for reaching 106 spores per ml of fungal suspension.
0.1 ml of freshly grown culture (106 CFU/ml) of each microorganism was aseptically spread onto the surface of Mueller-Hinton Agar. Paper disks (6 mm) were impregnated with 50 μl of 100 mg/ml the extract and Nano-capsules containing 100 mg/ml of the extract. The plates were incubated at 37 °C for 24 h (bacteria), 25 °C for 48 h (yeast), and 25 °C for 72 to 96 h (mold). Finally, diameters (mm) of the zone of inhibition were measured [16-18].
Micro Dilution method:
The microdilution method was used for determining the MIC of extract and Nano-extract. 100 μl of different concentrations (200, 100, 50, 25, 12.5, 6. 25, 3.125, and 1.765 mg/ml) of the extract and Nano-extract were added in each well of ELISA 96 well-plate, which contained 80 μl Mueller-Hinton broth. Then, 20 microliters of 106 CFU/ml suspensions of microorganisms were added to the wells. The negative control was prepared with MHB medium containing the extract or Nano-extract without the microorganism and the positive control was prepared with the MHB medium containing microorganisms without the extract or Nano-extract. ELISA plates were incubated at 37 °C for 24 h for bacteria, 25 °C for 24 h for yeast, and 25 °C for 48 h for mold. The turbidity of Microorganisms growth was detected by an ELISA reader. After the incubation period, the lower concentration of the extract or Nano-extract that had turbidity similar to negative wells was defined as MIC.
For detection of MBC/MFC, 100 μl of the well with turbidity similar to the negative control, was spread on the surface of MHA medium and incubated at suitable temperature and time regarding the microorganisms. The minimum bactericidal or fungicidal concentration was the lowest concentration of the extract or nano-extract that caused cell death of microorganisms and not observed any colony of microorganisms [16-18].
FINDINGS
Results of Disc Diffusion Method:
As shown in Fig. 1, the effect of nano-extract against yeast and Gram-positive and negative microorganisms was higher than extract. However, neither the extract nor nano-extract affected the growth of Aspergillus parasiticus. Regarding the diameter of the inhibition zone of microorganisms, the highest inhibition zone belonged to Candida albicans by 35 and 32 mm for nano-extract and extract respectively. Also, the result showed that the effect of the aerial organs of Hypericum Perforatum extract (zone of inhibition=15 mm) and nano-extract (zone of inhibition=21mm) on Staphylococcus aureus as a Gram-positive bacteria was higher than Escherichia coli as a Gram-negative bacteria. The inhibition zone of the extract and nano-extract against E. coli was 12 and 18 mm, respectively.
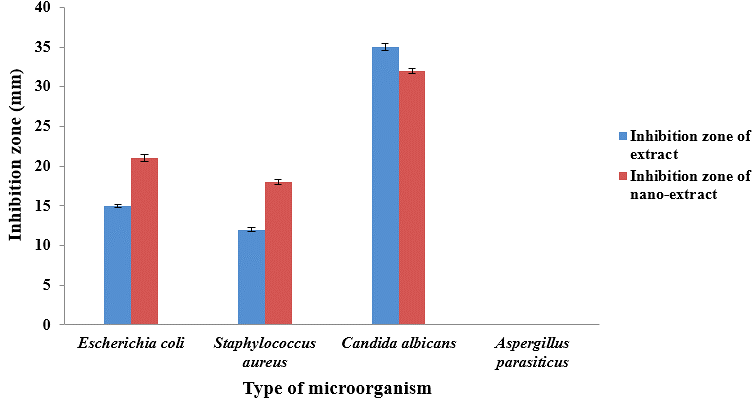
Figure 1. The effect of extract and nanoparticles containing the extract of the inhibition zone
of four groups of microorganisms.
The results of the Micro Dilution Method:
The antimicrobial activity of the extract and nano-extract was also evaluated by determining the minimum growth inhibitory concentration (MIC) and minimum bactericidal or fungicidal concentration (MBC/MFC) on several indicator microorganisms in food (Table 2). The results showed that the extract and nano-extract had an antimicrobial effect on all microorganisms tested in this study (Escherichia coli, Staphylococcus aureus, and Candida albicans) except Aspergillus parasiticus. The lowest concentration of extract to prevent the growth of Escherichia coli, Staphylococcus aureus, and candida albicans was 100, 50, and 25 mg/ml respectively. While, the MIC of nano-particle containing extract on Escherichia coli, Staphylococcus aureus, and candida albicans was 12.5, 6.25, and 3.125 mg/ml, respectively.
The results of MBC obtained from the effect of extract and nano-extract on the tested microorganisms are shown in Table 2. Similarly, to the MIC results, the obtained minimum bactericidal and fungicidal concentrations were different for microorganisms. By using Nano-extract, the lowest MBC/MFC of 3.125 mg/ml was obtained for Candida albicans, followed by Staphylococcus aureus (12.5 mg/ml) and Escherichia coli (6.25 mg/ml). Also, the MBC/MFC of the extract for these microorganisms was the same as MIC.
Table 2. MIC and MBC/MFC of extract and Nano-extract against four groups of microorganisms
|
|
Minimum Inhibitory Concentration (mg/ml) |
Minimum Bactericidal/Fungicidal Concentration (mg/ml) |
||
|
Microorganism |
Extract |
Nano-extract |
Extract |
Nano-extract |
|
Escherichia coli |
100 |
12.5 |
100 |
12.5 |
|
Staphylococcus aureus |
50 |
6.25 |
50 |
6.25 |
|
Candida Albicans |
25 |
3.125 |
25 |
3.125 |
|
Aspergillus parasiticus |
>200 |
>200 |
>200 |
>200 |
DISCUSSION:
The antimicrobial properties of plant extracts have often been attributed to its bio-active compounds. The type and amount of compounds affect the antimicrobial power of these extracts. Also, each bio-active compound is able to prevent the growth of special microorganisms. Examination of the cell structure of microorganisms by electron microscopy after exposure to antimicrobial plant extracts has shown changes in the membrane and cell wall structure, changes in the space between the wall and the membrane, and a decrease in cytoplasm volume [19].
The type of solvent using in extraction has a significant effect on the type and quantity of the extracted bio-compounds. The use of two solvents in extraction makes it possible to use the power of both solvents in the extraction of bioactive compounds with different polarities and increase extraction efficiency. Research has shown that alcohol increases the speed and efficiency of extraction due to the destruction of the cell wall and the increase in the availability of soluble substances[20].
Nano-encapsulation of sensitive bioactive compounds can preserve and enhance their physicochemical functionalities. Also, the release of these sensitive materials is controlled [21]. Alginate is a versatile biopolymer commonly used for the encapsulation of different compounds, due to its non-toxicity, availability, low cost, and easy to gel properties. Encapsulation of different plant extracts in calcium alginate has been investigated and the results showed the suitable efficiency of this method [22, 23].
Some researchers have isolated hyperforin as an antibacterial active constituent of the nonpolar extract of H. Perforatum [24, 25]. However, it was emphasized that the antibacterial effect of hyperforin is only observed at high concentrations [25]. Avato et al. (2004) investigated the antimicrobial effect of different extracts of H. Perforatum against various microorganisms. The ethyl acetate sub-extracts, including hyperforin, hypericin, and flavonoids are the main and most active constituents of this extract [26]. In addition, xanthones from H. Perforatum can impair the growth of pathogens [27].
Suntar et al. (2016) evaluated the antimicrobial activity of the flowering aerial parts of H. Perforatum by extraction with ethanol and then, this extract was fractionated to obtain 5 sub-extracts in different polarities. Resazurin microplate, modified microtiter-plate, and colorimetric micro-well dilution assays were used to assess antimicrobial activities against Streptococcus mutans, L. plantarum, Streptococcus. sobrinus, and Enterococcus. Faecalis. The results showed that the aqueous extract was more efficient than the other sub-extracts against all tested microorganisms at MIC values of 8–32 mg/mL and this sub-extraction method displayed strong activity against L. plantarum and S. sobrinus [28].
There is no evidence about the effect of antimicrobial activity of the nano-particle of Hypericum, but Sadrnia et al. (2017) compared Hypericum extract and its micro-emulsion on Gram-negative bacteria. Their finding confirmed that micro-emulsion in lower concentrations had the antibacterial effect equal to the aqueous extract. Salmonella typhi has the highest zone of inhibition around 42 mm at a concentration of 8 mg/ml for extract and 30 mm in 0.1 mg/ml of micro-emulsion [29].
In this case study, the antimicrobial effect of the extract and nano-capsules containing the hydroalcoholic extract of the aerial parts of Hypericum Perforatum on microorganisms showed that the antimicrobial effect of extract of nano-particles containing extracts on Candida albicans, Staphylococcus aureus, and Escherichia coli was better than the extract. Extract and nano-extract had no effect on Aspergillus parasiticus. Also, according to the obtained results, it seems that the conversion of the extract into nano-extract increases the effectiveness of the extract. It seems that in order for the extract to be effective, nano-extract on Aspergillus parasiticus needs to be used in larger quantities.
CONCLUSION:
The results of this study showed that both hydroalcoholic extract and Nano-extract of Hypericum Perforatum had a good antimicrobial effect on Candida albicans, Staphylococcus aureus, and Escherichia coli, but they did not affect Aspergillus parasiticus. Also, converting the extract to the nanoscale can increase the effectiveness of the extract containing antimicrobial compounds. It is recommended that this nanoscale structure be used for clinical trials.
REFERENCES