



|
Green-Synthesized Copper Nanoparticles Decorated on Ceria for Antibacterial and Treatment of polluted compounds
Nguyen P. Anh 1, Duong H. T. Linh 1, Hoang T. Cuong 1, Nguyen T. T. Van 1, Ngo M. Trung 2, 3, Pham N. A. Vi 2, 3, Huynh X. Thuong 4, Nguyen V. Minh 4, Nguyen Tri 1, 4* |
|
1 Institute of Chemical Technology, Vietnam Academy of Science and Technology, 01 Mac Dinh Chi Street, Ho Chi Minh City, Vietnam. 2 Vietnam National University Ho Chi Minh City, Linh Trung Ward, Thu Duc District, Ho Chi Minh City, Vietnam 3 Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, District 10, Ho Chi Minh City, Vietnam. 4 Ho Chi Minh City Open University, 97 Vo Van Tan Street, District 3, Ho Chi Minh City, Vietnam. |
|
ABSTRACT |
| A multifunctional material of 7.5 wt.% CuNPs decorated on CeO2 (7.5Cu-Ce) was synthesized by the green method using Cocoa pod extract as a reducing agent. The properties of the material were studied by various techniques including N2 physisorption (BET), X-ray diffraction (XRD), Raman spectroscopy (Raman), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), and high-resolution transmission electron microscopy (HRTEM). In the next step, to investigate the applications of the material, it was screened for antibacterial activity against clinically important bacteria such as E. coli and Salmonella. The results showed high antibacterial activity, the minimum inhibitory concentrations (MIC) for E. coli and Salmonella are similar and reached 312.5 g.mL1. Also, the catalytic activities of the material were studied in the deep oxidation of CO or/and p-xylene. The conversion of CO was much easier than that of p-xylene. The CO conversion in the mixture is much lower than that of the pure substance. On the contrary, in the mixture of CO + p-xylene, the conversion of p-xylene is much higher compared to the pure p-xylene. |
|
Key Words: copper nanoparticles, green synthesis, Cocoa pod extract, antibacterial, catalytic oxidation. |
INTRODUCTION
Nanotechnology plays an important role in modern research, relating to the synthesis of nanoparticles of varying shapes, sizes, and chemical compositions and their possible applications for human benefits [1-5]. The technology can be applied in a wide range of fields, especially in medicine and chemical industries [6-8]. Nanoparticles are relevant for catalytic activity and other related properties such as their antibacterial activity [9, 10]. The synthesis, as well as the use of the metal nanoparticles, have gained consideration due to their unique magnetic, electrical, optical, catalytic and antimicrobial properties [11]. Nanoparticles can be synthesized using various methods, such as chemical, physical, biological, etc [12].
In recent years, green synthesis has been one of the best methods for synthesizing nanoparticles [13, 14]. Green chemistry refers to the development and design of chemical products and processes to minimize the dangerous uses of the environment [15]. In this method, the plant extracts including stem, flower, fruit, leaves, and bark are used as a reducing and stabilizing agent for nanoparticles [14]. Various metal nanoparticles (silver, gold, copper, platinum, palladium, etc) were synthesized using the green synthesis method [16-20]. Among metal nanoparticles, copper nanoparticles (CuNPs) have attracted great attention from researchers for the past few decades due to their low-cost and electrical, optical, and catalytic properties [21-23]. CuNPs are also widely investigated in various medical, antifungal, and antibacterial applications [24-26]. The main challenge in the development of CuNPs was to prepare nanomaterials that are highly active, selective, stable, and inexpensive. One economical way of obtaining innovative Cu-based nanomaterials is to anchor CuNPs on various supports such as SiO2, CeO2, TiO2, etc [27]. Over the last few decades, ceria has been applied in many fields due to its unique redox properties and high oxygen storage capacity due to the facile redox cycle between the Ce3+ and Ce4+ in the structure [28]. In particular, much attention has been paid to copper-doped ceria based material because of their unique activity.
In our unpublished study, CuNPs were synthesized by green chemistry using the Cocoa pod (CCP) extract as a reducing agent. The results confirmed that the proteins and phenolics compounds in cocoa pod extract can be acted both as the reducing agents for the formation of CuNPs. The suitable conditions for the synthesis of copper nanoparticle using CCP extract as reducing agent were determined such as the volume ratio of Cu(NO3)2 solution/CCP Extract of 3.5/1.5, stirring rate of 300 rpm, pH solution of 7.5, the temperature of 75 °C and the synthesis duration within 180 minutes. The CuNPs had a highly crystalline nature with an average size of 34.4 nm. Besides, the CeO2 had positive influences on the dispersibility of CuNPs, thereby reducing the particle size of CuNPs. The Cu–Ce catalyst showed the highest activity in benzene oxidation and its activity is much higher than that of the pure CuNPs with the same content supported on TiO2 and Al2O3. It was interesting to found that the 7.5 wt.% of CuNPs decorated on CeO2 exhibited the best catalytic performance towards benzene oxidation. However, their antibacterial activity, as well as their oxidation capacity in the mixture, have not been investigated. In this study, 7.5 wt.% of CuNPs supported on CeO2 (7.5Cu-Ce) was synthesized using CCP extract as a reducing agent. Antibacterial activity of the 7.5Cu-Ce material was assessed against bacteria such as E. coli and Salmonella. Also, the catalytic activity of the material was investigated in the deep oxidation of CO or/and p-xylene.
EXPERIMENTAL
Materials
Cocoa pods were collected from Tien Giang province, Vietnam. After washing and draining, 50 g of the Cocoa pods were minced and mixed with 1000 mL of deionized water. Then, the mixture is heated to 80 °C for 2 hours under stirring. Finally, the Cocoa pod extract (CCP extract) was filtered and preserved at 4 °C until further experiments. Copper nitrate (Cu(NO3)2.6H2O, > 99.8%), starch ((C6H10O5)n, > 99%) and sodium hydroxide (NaOH, > 99.9%) were purchased from Merck.
Preparation
The green synthesis of 7.5 wt.% of CuNPs supported on CeO2 (7.5Cu-Ce) was started by adding 1.5 grams starch and 0.276 g CeO2 nanorods to the 35 mL of 0.02 M Cu(NO3)2 solution with vigorous stirring at 300 ppm. The solution color changes from blue to white in this step. Then, 15 mL of CCP extract was added to the mixture. The color transformation from white to blue moss occurred in the aqueous phase. NaOH solution (0.1 M) was added to the reaction mixture under continuous vigorous stirring to adjust pH = 7.5. The mixture was heated to a temperature of 80 oC and then kept for 3 hours. The formation of the copper nanoparticles on CeO2 is confirmed by the color changing from green to brown. After completion of the reaction, the sample was cooled to room temperature and the suspension was centrifuged. The resulting precipitate was filtrated and washed with distilled water three times. Finally, the obtained copper nanoparticles on CeO2 were dried at 60 °C overnight.
Characterization
X-ray powder diffraction (XRD) patterns were collected on Bruker D2 Phaser diffractometer using CuKα radiation (λ = 0.154 nm) in 2θ = 10–80° with the scanning step of 0.02°. The average crystal size of copper nanoparticles, d (nm), was calculated according to the Scherrer equation [29]:
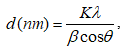 , (1)
, (1)
where K, the Scherrer constant, is taken to be 0.94, λ is the wavelength of the X-ray, b is the line width at half maximum height of the peak in radians, and θ is the position of the peak in radians. Fourier transform infrared spectroscopy (FT-IR) carried out on a Tensor 27-Bruker spectrophotometer operating in the range of 400–4,000 cm-1 at a resolution of 2 cm-1. Raman spectroscopy measurements were carried out at room temperature with a laser Raman spectrometer (Invia, Renishaw). The morphology and surface properties of materials were investigated by a Hitachi S4800 field emission scanning electron microscopy (SEM) apparatus. The particle size and the crystal phases were also estimated by HRTEM analysis on JEOL 2100F instrument. Nitrogen adsorption-desorption isotherms were determined by using a Nova 2200e instrument. The specific surface area of samples was calculated according to the Brunauer-Emmett-Teller (BET) nitrogen adsorption isotherms.
Antibacterial activity
To examine the minimum inhibitory concentration of 7.5Cu-Ce against E. coli and Salmonella, the different concentrations of 7.5Cu-Ce nanoparticles (N, N/2, N/4, N/8, N/16, N/32 and N/64 with N was the initial concentration of the 7.5Cu-Ce solution in deionized water, N = 5 mg/mL) were prepared by diluting the 7.5Cu-Ce solution with deionized water. Subsequently, the diluted samples were mixed with the sterile nutrient agar. By using sterile sticks, the standardized inoculum of each selected bacteria with 1.5×10^7 CFU/ml was inoculated on agar plates mixed with 7.5Cu-Ce samples from low to high concentrations. A plate of the sterile nutrient agar was not mixed with 7.5Cu-Ce nanoparticles for control [30]. Each strain of bacteria was inoculated at a point on a disk with the same location on the disks. Finally, the plates were incubated at 37 °C for 24 hours. The lowest concentration of AgNPs that inhibits the growth of tested bacteria was considered as the minimum inhibitory concentration (MIC) [31].
Catalytic activity in deep oxidation
The catalytic behavior of samples expressed as the conversion of AHs was measured in a fixed tubular quartz reactor (i.d. 8 mm) under atmospheric pressure at a temperature range of 75 to 400 oC. The weight hourly space velocity (WHSV) was 60,000 mL.h-1.g-1, and the mass of material was 0.2 grams with a size range of 0.25 to 0.50 mm. The inlet concentrations of CO, p-xylene, and oxygen in nitrogen were 0.50, 0.34 and 10.5 mol%, respectively. The reactant and product mixtures were analyzed with an Agilent 6890 Plus Gas Chromatograph with an FID detector and capillary column DB-624 and with a TCD detector and capillary column TG-BONQ. The tests were conducted in triplicate to ensure the accuracy of the results. The CO and p-xylene conversion were calculated as follows:
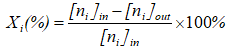 , (2)
, (2)
where Xi (%) - percent AHs conversion of “i” compound; i - CO or p-xylene; [ni]in and [ni]out - the input and output mole of “i” compound, respectively.
RESULTS AND DISCUSSIONS
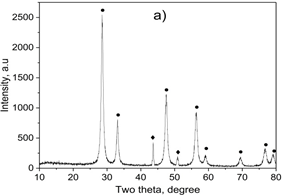
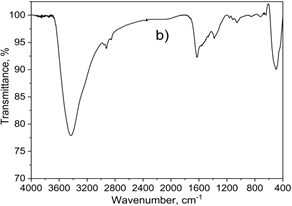
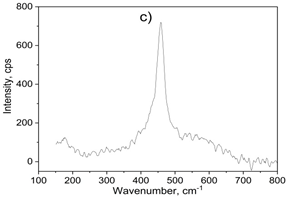
Figure 1. XRD diffraction pattern (a), FT-IR (b) and raman spectum (c) of 7.5 wt.% CuNPs decorated on CeO2 sample
X-ray diffraction analysis (XRD) result of the samples was shown in Figure 1a. Typical diffraction peaks for fluorite structured CeO2 were observed at 2θ of 28.7, 33.1, 47.5, 56.3, 59.1, 69.4, 76.7, and 79.1° (JCPDS 43-1002). Two diffraction peaks for the cubic structure of metallic copper were observed at 2θ of 43.6 and 50.7 corresponding to (111) and (200) lattice planes, respectively (JCPDS card No. 04-0836). Therefore, XRD confirmed a highly crystalline nature of the sample. Based on the XRD highest peak of at 2θ = 28.7° and 43.6°, the average crystal size of CeO2 and CuNPs was determined following the equation the Scherrer equation. The mean particle size calculated from the XRD patterns is 34.4 and 24.5 nm, respectively. According to our previously unpublished report, the average particle size of the pure CuNPs was 34.4 nm. The formation of CuNPs on the CeO2 support was the cause of reducing the agglomeration of CuNPs particles, leading to reducing the size of CuNPs particles.
FT-IR spectra of 7.5Cu-Ce were described in Figure 1b. FT-IR spectrum manifested strong peaks at 3295, 2850, and 1640 cm-1, implicating proteins and phenolic compounds as the reducing and stabilizing agents. In which, the band at 3295 cm-1 was typical of the N–H bond of amines [32, 33]. The C-H stretching was also observed at 2850 cm-1. The C=C stretch of alkenes or C=O stretch of amides were detected at 1640 cm-1 [33]. On the other hand, a band centered at 2285 cm-1 was related to the Cu-H stretching [34], and the peaks at 640 and 530 cm-1 corresponded to Cu-O bond, indicating the formation of the CuNP. Besides, the absorption band at 450 cm-1 corresponds to CeO2 stretching vibration [35].
Raman spectroscopy allows probing the presence of oxygen defects in ceria-based materials. It can be found that the asymmetrical band was exhibited on the sample at 465 cm-1 correspondings to the degenerated F2g active mode of fluorite-type structure CeO2 (seen in Figure 1c) [36]. Besides, the weak band at 595 cm-1 attributed to the presence of Frenkel-type anion defects in the ceria lattice [37].

Figure 2. SEM image of a sample
Scanning electron microscopy (SEM) was used to obtain the morphology of the 7.5Cu-Ce nanocomposite. From the SEM image of the 7.5Cu-Ce (Figure 2), it can be seen that the nanorods of CeO2 were formed with the length range from 100 to 200 nm, and the diameter range from 20 to 30 nm. The SEM image also shows the formation of cubic particles of CuNPs. HRTEM analysis was performed to investigate the microstructural characteristics of the catalysts. The HRTEM image of the 7.5Cu-Ce (Figure 3) confirmed the thermal stability of CeO2 nanorods after Cu addition and calcination at 450 oC for 1 hour in N2. The CeO2 nanorods were 20-30 nm in diameter and 100-200 nm in length, which is consistent with the previous SEM analysis. The inset in Figure 3 demonstrated that CeO2 nanorods exposed both (100) and (110) planes of structured fluorite CeO2 with spacings of 0.25 and 0.27 nm, respectively. Besides, the HRTEM image analysis also indicated that the formation of cubic particles of the CuNPs was exposed to CeO2 nanorods. The estimated d-spacings of 0.25 and 0.29 nm (inset above Figure 3) were assigned to (111) and (110) crystal planes of CuNPs phase in 7.5Cu-Ce sample, respectively. The particle size of CuNPs has estimated in the range of 20-30 nm. This is entirely consistent with the previous XRD analysis of the sample.
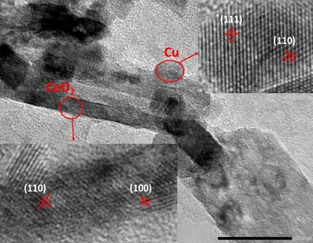
Figure 3. HRTEM image of a sample
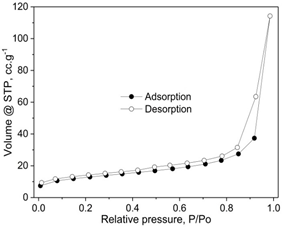
Figure 4. N2 adsorption/desorption isotherms of a sample.
The nitrogen adsorption/desorption isotherm of the 7.5Cu-Ce catalyst are plotted in Figure 4. The sample presented classical type IV isotherms with well-denied H2-type hysteresis loops, confirming the formation of mesoporous materials according to IUPAC classification [28, 38]. The H2 type of hysteresis loop refects a complex pore structure, including typical in pot shaped pores, tubular holes with an asymmetrical distribution of pore diameter and the mesopores of the closely packed spherical particle, etc.
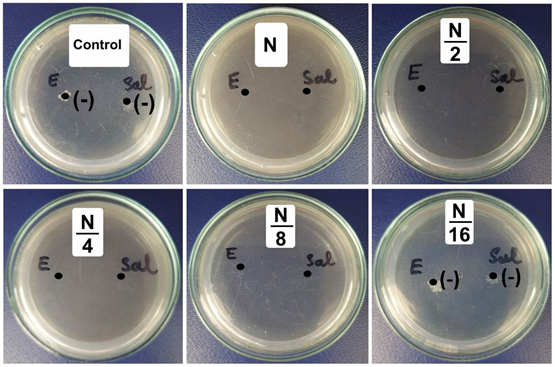
(-): No antibacterial
Figure 5. The minimum inhibitory concentrations of 7.5 wt.% CuNPs decorated on CeO2 against E. coli (E), B. cereus (C), and B. subtilis (S).
The antibacterial activity of the 7.5Cu-Ce sample against E.coli and Salmonella was clearly shown in Figure 5. The results showed high antibacterial activity, the minimum inhibitory concentrations (MIC) for E. coli and Salmonella are similar and reached 312.5 g.mL1. The potent antibacterial properties of 7.5Cu-Ce may be attributed to the released metal ions, which could have interaction with microorganisms using their attaching to the surface of the cell membranes of bacteria and penetrating the bacterial cells.
Katrin et al used the Capparis spinosa extract as a reducing agent to synthesize the copper nanoparticles and evaluated their antibacterial activities against S. aureus, B. cereus, K. pneumoniae, and E. coli [32]. The results showed the effect of CuNPs on the gram-positive bacteria was greater. The MIC values of copper nanoparticles against S. aureus, B. cereus, K. pneumoniae, and E. coli reached 5, 5, 10 and 10 mg.L-1, respectively. Jayesh et al investigated the antibacterial properties against designed strains of copper nanoparticles prepared by wet chemical synthesis involving the stoichiometric reaction between sodium borohydride and copper ions [39]. The MIC values against E.coli and S. aureus were determined in a range of 140-240 mg.mL-1. It can be found that synthesized 7.5Cu-Ce using CCP extract as a reducing agent exhibited highly great bactericidal activity. This can be that the formation of CuNPs on the CeO2 support led to reducing the size of CuNPs particles, resulting in enhancing antibacterial activities of CuNPs.
The catalytic activity of 7.5Cu-Ce in the treatment of p-xylene or/and carbon monoxide are represented in Figure 6. The results in Figure 6a showed that the conversion of the pure CO is 12.7% started at 75 °C and completed at 125 °C. Meanwhile, the conversion of pure p-xylene just can be completed at a temperature of 400 °C in Figure 6b. The conversion of CO is much easier than that of p-xylene. It was explained that the p-xylene is a cyclic hydrocarbon, so it is more difficult to oxidize than CO. On the other hand, the completions of CO and p-xylene conversion were performed at 225 and 375 oC with a mixture of CO and p-xylene, respectively (seen in Figure 6c). For comparison purposes, T50 and T90 (the temperatures at which the CO/p-xylene conversion reaches 50 and 90% is taken as a measurement of catalytic activity) in each experiment had been established (seen in Figure 6d). The conversion of CO in the mixture is much lower than that of the pure substance, where T50 and T90 were 196 and 209 oC compared with 91 and 115 oC, respectively. On the contrary, in the mixture, the conversion of p-xylene is much higher compared to the pure p-xylene (247 and 281 oC versus 263 and 304 oC). This proves that the presence of CO hardly affects the oxidation of p-xylene in the mixture.
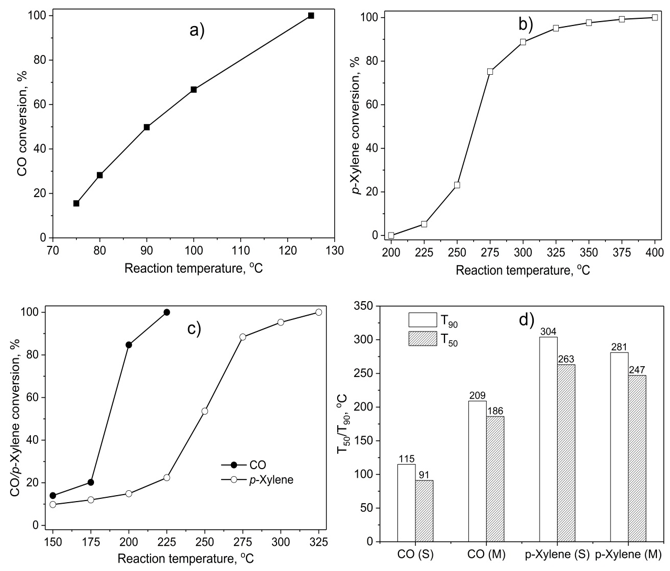
Figure 6. The catalytic activity of 7.5 wt.% CuNPs decorated on CeO2 in the removal of p-xylene or/and carbon monoxide; a) the removal of single CO; b) the removal of single p-xylene; c) the removal of CO + p-xylene mixture; d) the comparison of the temperatures at which the CO/p-xylene conversion reaches 50% (T50) and 90% (T90) between single oxidation (S) and mixture oxidation (M).
Balzer et al. synthesized Co2O3/CeO2-γ-Al2O3 catalysts for the deep oxidation of VOCs including n-hexane, benzene, toluene and o-xylene [40]. The sample with 20 wt.% Co2O3 was the best one for VOCs deep oxidation, and conversion of n-hexane, benzene, toluene, and o-xylene reached 96, 90, 70 and 58% respectively at a reaction temperature of 350 °C. Meanwhile, 20 wt.% Co2O3/γ-Al2O3 undoped CeO2 just reached 50, 45, 35 and 25% under the same conditions. Wu et al. prepared Au catalyst loaded (1.5 wt.%) on the different supports (ZnO, Al2O3 and MgO) by a colloidal deposition method for the oxidation of p-xylene [41]. The p-xylene conversion achieved 30, 14 and 9% respectively at a reaction temperature of 300 oC on Au/ZnO, Au/Al2O3 and Au/MgO catalysts. When comparing the activity of 7.5Cu-Ce catalyst with others, it can be found that this catalyst had also a higher catalytic activity, oxidizing more than 90 % of p-xylene to CO2 at 350 °C.
CONCLUSION
In this investigation, the 7.5Cu-Ce nanoparticles were synthesized through an eco-friendly approach using CCP extract as a reducing agent. This approach has many advantages such as simple and the process can be scaled up with economic viability. The formation of CuNPs on the CeO2 support was the cause of reducing the agglomeration of CuNPs particles, leading to reducing the size of CuNPs particles. The 7.5Cu-Ce nanoparticle showed significant antimicrobial activity on selected pathogenic bacteria. Besides, it also showed efficient performance for CO and/or p-xylene deep oxidation. These results demonstrated that the 7.5Cu-Ce nanocomposite is a good candidate for antibacterial and removal of CO and p-xylene at low-temperature.
ACKNOWLEDGEMENT
The study was supported by The Youth Incubator for Science and Technology Program, managed by Youth Development Science and Technology Center - Ho Chi Minh Communist Youth Union and Department of Science and Technology of Ho Chi Minh City, the contract number is "22/2019/HĐ-KHCNT-VƯ".
REFERENCES