



|
Evaluation the Effect of New Transdermal Oxygen Therapy Method on Healing of Pressure Ulcer in Children |
|
Mehdi Ahmadinejad1, Morteza Hashemian2, Zahra Noradini3 , Maryam Ahmadipour4* |
|
|
|
1associated Professor, Fellowship of Critical Care, Faculty of Medicine, Department of Anesthesia, Shahid Bahonar Hospital, Kerman University of Medical Sciences, Kerman, Iran. 2 Assistant Professor, Fellowship of Pain ,Faculty of Medicine, Department of Anesthesia, Shahid Bahonar Hospital, Kerman University of Medical Sciences ,Kerman ,Iran. 3 Masters Degree of Critical Care Nursing,Payambar Aazam Hospital, Kerman, Iran. 4Assistant Professor, Pediatric Cardiologist, Faculty of Medicine, Department of Pediatric, Kerman University of Medical Sciences ,Kerman, Iran. |
ABSTRACT
Introduction: One of the important duties of nurses in ICUs is to take of skin problems in patients and to cooperate in the healing of bedsores (also called pressure sores or ulcer sores). Despite recent advances in the treatment of bedsores, they are still one of the major problems ICU patients face, and an important cause of death in these patients, in addition to putting a heavy financial burden on patients and hospitals. In fact, about 7-8 percent of cases of death in ICU patients are related to pressure sores. Materials and methods: This double-blind clinical trial was conducted on 80 pediatric(2-18 years) ICU patients with stage 3 bedsores. The control group received regular care including daily washing with normal saline followed by Comfeel ulcer dressing, while the intervention group received 20-minute humidified oxygen treatment at 10 liters per minute twice per day every via a transparent sterile cover which had not any contact to sore in addition to the regular care. The wounds were assessed at the end of the first and second weeks with respect to granulation tissue formation, surface area of the wounds, and the extent of exudation. The data was analyzed using SPSS version 19 and repeated measures ANOVA. Findings: The average age of the patients in the two groups was 65/6 ±9/14 years. At the end of the second week, the following results (which were all significantly different between the intervention and control groups at the 0.0001 level) were observed: the surface areas of the wounds were 3.56 ± 2.47 in the intervention group and 5.7 ± 2.1 in the control group, the degrees of exudation were 1.07 ± 0.27 in the intervention group and 1.55 ± 0.61 in the control group, the extents of granulation tissue formation were 0.99 ± 1.24 in the intervention group and 1.65 ± 1.04 in the control group, and the PUSH scores were 5.59 ± 3.6 in the intervention group and 8.90 ± 3.2 in the control group. Conclusions: The present research indicated that trans dermal oxygen therapy could reduce exudation and surface area of wounds and increase granulation tissue formation significantly in pediatrics.
Key Words: Pressure Ulcer, Trans Dermal Oxygen, Skin.
Pressure ulcers are among the most important problems of the patients in the ICU such that they are considered as good indicators of the health care quality [1]. This complication is caused by the decreased blood supply to the soft tissue following the compression of two hard surfaces, such as bone surfaces from the inside and the surface of the bed or chair from the outside, and the necrosis cells cause destruction of the adjacent cells [2]. In the United States, about 1 to 1.7 million cases of pressure ulcers are reported annually, but in general, there is still no accurate data on the prevalence of pressure ulcers in the ICU and only a few studies reported the prevalence in the wards as 5% and in the ICUs as 10 to 20% [3]. In addition to the psychological and mental complications on the patient’s companion, pressure ulcers can cause pain, infection, bleeding and even death. Osteomyelitis, sepsis, and cellulitis with associated malodorous drainage are among the pressure ulcer complications that can ultimately lead to death [4]. Moreover, the medical expenses of these ulcers types are very high as in the United States, about 2 billion and 200 million dollars are spent annually on the treatment of pressure ulcers, likewise much time is spent on its treatment [5]. On average, the hospitalization time increases by eight days for the patients with Stage 2 pressure ulcers [6]. There are several underlying causes for pressure ulcers among which the following can be referred: organs failure, smoking, dark-skinned ethnicity, age over 70, male gender, immobility, urinary and faecal incontinence, neurological diseases, dry skin, fever, low systolic and diastolic blood pressure, heart disease, pulmonary disease, diabetes, infection, fraction, quadriplegia, alcoholism, dehydration, malnutrition, inadequate protein and calorie intake, and hypoalbuminemia. Specific risk factors of ICU including respiratory failure are dependent on mechanical ventilation [7, 8]. Old age, inappropriate nutrition, excessive sweating and decreased level of consciousness are among the important risk factors [9]. People admitted to the ICUs, are the ones with spinal cord injuries and the elderly are in the high risk groups [10]. Sacrum, coccyx, heel, elbow, knee, ankle and back of the skull are susceptible to pressure ulcer [11]. The most frequently involved region in pressure ulcer is sacrum with 30-49%, followed by heel with 19-36%, and the pelvic region with 6% [12, 13]. Wound healing is a complex biological process that has four distinct phases including tissue homeostasis, inflammation, proliferation, and reconstruction. The main cause of the healing process is angiogenesis, which results in the saturation of blood vessels and provides a suitable environment for wound healing; moreover, macrophages and fibroblasts, as the key regulator of angiogenesis, at the wound site are not useful without oxygen [9]. Despite widespread research and new treatment methods of pressure ulcer, the wound healing process is still slow, which causes many problems for the patients.
Oxygen, as the most abundant element in the Earth's atmosphere, is an integral part of the survival of aerobic cells [14]. Prior to this, local oxygen therapy has been used to treat various types of ulcers, and it has been observed that by increasing mitochondrial oxidative activity, oxygen can lead to production increase of ATP and protein, angiogenesis and wound reconstruction, ultimately resulting in accelerated wound healing [15, 16]. However, most of the studies have been conducted using hyperbaric oxygen as Sultan et al., (2010), showed that the use of hyperbaric oxygen at the site of venous stasis ulcer can reduce the severity of the pain, relapse, and wound surface area [17, 18]. Moreover, in a study by Wick et al. (2011) conducted in the Wound Care Clinic at Boston University, it was found that the use of local oxygen therapy in diabetic wounds can reduce wound secretions, wound surface area, and levels of inflammatory cytokine and protease [19]. Since the ICU therapeutic team are responsible for the care and treatment of pressure ulcer and given the probability that local oxygen therapy might be useful in wound healing and because of the high cost and the limitation of using hyperbaric oxygen in the ICU, and using the available tools, we tried to examine the effect of pure oxygen at normal pressure on the pressure ulcer treatment process in the trauma patients in the ICUs.
MATERIALS AND METHODS
This study is a double blind clinical trial with the aim of investigating the effect of local oxygen effect on the pressure ulcer treatment in 2 to 18-year old children admitted to the TICU. Having obtained the ethical code of IR-KMU-REC.1395.18 from Kerman University of Medical Sciences research deputy and registered on the Iranian Registry of Clinical Trials database (IRCT201602275426N9), the written informed consent was obtained from the legal guardians of the patients admitted to the TICU at Shahid Bahonar Hospital in Kerman and the research began in the middle of 2016.
The inclusion criteria included: 2 to 18-year old patients with GCS<8 due to the severe traumatic brain injury, having stage 3 pressure ulcers and completing the informed consent by the legal guardian. The exclusion criteria included: the legal guardians’ unwillingness, organ failure, diabetes, burn, history of smoking, drug or alcohol addiction, GCS<8, patient death during the study, systemic corticosteroid consumption over the last year, chronic skin disease, organ failure, diarrhea, vascular problems, and hypoxic patients. In case the pressure ulcers had the infectious symptoms at the onset of admission including inflammation, redness around the wound, malodorous and purulent drainage, the patients with hypoalbuminemia, and cachexia symptoms (including wrinkled skin on the back of the arm, the patient’s physical status, protruding of zygomatic arch, and hollowness of temporal cavity) as well as for monitoring the patients’ nutrition, urea balance test was used and in case of being negative, the amount of calorie was calculated by a nutrition consultant and the problem was solved.
The sample size, regarding the type 1 error at the level of 0.05, power of 0.8, and the standard deviation of the push score according to the previous studies [20], was considered as 80 patients (40 patients in each group) that were randomly assigned to the groups. The control group (40 patients) received only regular care such as daily washing with normal saline and the common Comfeel dressing; and the case group (40 patients), in addition to the regular care, received local oxygen at flow rate of 10 liters per minute for 20 minutes twice a day through sterile coating that covered the entire ulcer but did not have a direct contact with the wound surface.
At the beginning of the study and at the end of the first and second weeks, the wound was examined by a trained person who was unaware of the wound treatment method and the wound status was recorded according to the push scale. We used a sterile scaled spatula to measure the wound surface area and examine the amount of wound drainage with the following criteria:
Score 2: drainage in a moderate amount (one gauze pad is wet)
We examined tissue healing with the following criteria:
The highest score in push system is 17 and the lowest score is 3. The data with regard to age and gender, stage of ulcer, wound surface area, and amount of drainage were collected from all the patients. Before the intervention, each wound was examined completely. After data collection, they were analyzed using SPSS19. Data were reported by frequency, percentage, and central tendency and dispersion. We used Chi-square test and t-test to compare the intervention and control groups in terms of demographic variables. We used push scores obtained from variance analysis through repeated measure ANCOVA by controlling the pre-intervention scores in order to compare the intervention and control groups in terms of the variables of wound surface area, drainage, and tissue type.
FINDINGS
Given the demographic information, 80 patients were included in the study that 67.5% were males and 32.5% were females; the male gender frequency in the intervention group was 70% and in the control group was 65%; there was no significant difference between the two groups in this regard (p=0.63). The patients were within the age range of 2-18 and the total mean age was 6.65±14.9 years; the mean age was 7.1±15.2 years in the intervention group and it was 6.2±14.6 years in the control group; and there was no significant difference between the two groups in this regard (p=0.19).
Prior to the study, the wound surface area was 1.7±7.9 cm2 in total population (1.9±6.87 cm2 in the intervention group and 1.7± 7.32 cm2 in the control group). There was no significant difference between the two groups. By the end of the first week, it was 1.7±5.48 cm2 in the intervention group and 1.8±6.49 cm2 in the control group, (P<0.0001). By the end of the second week, there was a significant difference between the intervention group 2.47± 3.56 cm2 and the control group (2.1 ± 5.7 cm2, P<0.0001).
The amount of drainage from wound was 0.55±2.42 in total population prior to the study (0.55±2.42 in the intervention group and 0.55±2.42 in the control group) and there was no significant difference between the two groups. By the end of the first week, it was 0.46±1.2 in the intervention group and 0.61±2.07 in the control group, (P<0.0001). By the end of the second week, there was a significant difference between the intervention group 0.27±1.07 and the control group (0.6±1.55, P <0.0001).
The occurrence rate of wound stages (as explained in the materials and methods part) was 0.82±2.48 in total population prior to the study (0.8±2.37 in the control group and 0.84±2.6 in the intervention group); the difference was not significant. By the end of the first week, it was 1.05±1.56 in the intervention group and 0.97±2.08 in the control group (P<0.0001). By the end of the second week, there was a significant difference between the intervention group 0.99±1.24 and the control group (1.04±1.65, P<0.0001).
The push score was 2.44±12.01 in total population prior to the study (2.5±11.67 in the control group and in the 2.3±12.35 in the intervention group). By the end of the first week, it was 2.6±8.23 in the intervention group and 2.84±10.66 in the control group (P<0.0001). By the end of the second week, it was 3.6±5.59 in the intervention group and 3.2±8.90 in the control group which means that the push score decreased in the intervention group compared to the control group (P<0.0001, Table 1).
By the end of the first week, the wound surface area was decreased in 92.5% of the patients in the intervention group, while the wound surface area was decreased in 62.5% of the patients in the control group. The difference was significant (P<0.0001). By the end of the second week, the wound surface area was decreased in 95% of the patients in the intervention group, while the wound surface area was decreased in 77.5% of the patients in the control group (P<0.0001). By the end of the second week compared to the first week, the wound surface area was decreased in 77.5% of the patients in the intervention group, while the wound surface area was decreased in 70% of the patients. The difference was significant (P<0.0001).
By the end of the first week, the amount of drainage in the intervention group was reduced by 92.5%, while the amount of drainage in the control group was reduced by 30% (P<0.0001). By the end of the second week, the amount of drainage in the intervention group was reduced by 97%, while the amount of drainage in the control group was reduced by 77.5% (P<0.0001). By the end of the second week compared to the first week, the amount of drainage in the intervention group had 12.5% reduction, while the reduction was 55% in the control group (P<0.0001); the difference was significant.
By the end of the first week, 77.5% of the patients in the intervention group had a back-staging in the ulcer stage (as explained in materials and methods part); while, 40% of the patients in the control group had a back-staging in ulcer stage (P<0.0001). By the end of the second week, 87.5% of the patients in the intervention group had a back-staging in the ulcer stage; while, 60% of the patients in the control group had back-staging in the ulcer stage, (P<0.0001).
By the end of the second week, 52.5% of patients in the intervention group had back-staging in the ulcer stage, while 40% of the patients in the control group had back-staging in the ulcer stage. The difference was significant (P<0/0001).
By the end of the first week, the push score in the intervention group was 97.5%; while, it was 77.5% in the control group (P<0.0001). By the end of the second week, the push score was decreased in 100% of the patients; while, the decrease was 92.5% in the control group (P<0.0001). In the second week compared to the first week, the push score was decreased in 87.5% of the patients; while, the push score was decreased by 92.5% in the control group and the difference was a significant (p<0.0001) (Table 2).
By the end of the first week, the wound surface area in the intervention group was decreased by 1.67 cm2 (22%) on average; while, the wound surface area in the control group was decreased by 0.55 cm2 (8%) (P<0.0001). By the end of the second week, the wound surface area in the intervention group was decreased by 3.60 cm2 (49%); while, it decreased by 1.37 cm2 (20%) in the control group (P<0.0001). By the end of the second week compared to the first week, it was decreased by 1.93 cm (34%) in the intervention group; while, it was decreased by 0.82 cm2 (13%) in the control group and the difference was significant (P<0.0001).
By the end of the first week, the amount of drainage was decreased by 22.1 degrees (50 %) in the intervention group; while, it was decreased by 0.35 degrees in the control group (14 %), (P<0.0001). By the end of the second week, it was decreased by 1.35 degrees (55%) in the intervention group; while, it was decreased by 0.87 degrees (36%) in the control group (P<0/0001). In the second week compared to the first week, it was decreased by 0.12 degrees (10%); while, it was decreased by 0.52 (25%) in the control group and the difference was a significant (P<0.0001).
By the end of the first week, the stage of wound tissue was decreased by 0.33 (35%) in the intervention group; while, it was decreased by 0.4% (16%) in the control group (P<0.0001). By the end of the second week, the stage of wound tissue was decreased by 1.5 (57%); while, it was decreased by 0.82 (34%) in the control group (P<0.0001). In the second week compared to the first week, it was decreased by 0.57 (34%) in the intervention group; while, it was decreased by 0.42 (21%) in the control group and the difference was significant (P<0.0001).
By the end of the first week, the push score was decreased by 3.8 (31%) in the intervention group; while, it was decreased by 1.3 (11%) in the control group (P<0.0001). By the end of the second week, the wound surface area was reduced by 6.45 (52%) in the intervention group; while, it was reduced by 3.07 (26%) in the control group, (P<0.0001). By the end of the second week compared to the first week, it was decreased by 2.62 (30%) in the intervention group, on average; while, it was decreased by 1.77 (17%) in the control group and accordingly the difference was significant (P<0.0001). (Table 3)
Given the repeated measure ANOVA test, the wound surface area in the intervention group was reduced more than the same in the control group. Moreover, the drainage, tissue type and push scores in the intervention group was decreased more compared to the control group, the results of which are represented in the table.
Table 1: Comparison of Control and Intervention Groups in terms of Variables of Wound Surface Area, Tissue Type, Wound Drainage, Pre and Post Intervention Push Scores of the Patients Admitted to the TICU Center Affiliated to Kerman University of Medical Sciences
|
Groups Variable
|
Treatment group Standard deviation ± Mean |
Control group Standard deviation ± Mean |
Test results Repeated measures ANOVA |
|
Wound surface area Pre-intervention First week Second week |
1.7±7.32 1.7±5.65 2.47±3.7 |
1.9±6.87 1.8±6.87 1.2±5.5 |
F=22 P<0.0001 |
|
Amount of drainage Pre-intervention First week Second week |
0.55±2.4 0.46±1.2 0.27±1.07 |
0.55±2.42 0.61±2.07 0.61±1.55 |
F=57.86 P<0.0001 |
|
Tissue stage Pre-intervention First week Second week |
0.84±2.6 1.05±1.67 1.24±1.1 |
0.8±2.37 0.97±1.97 1.04±1.55 |
F=14.23 P<0.0001 |
|
Push score Pre-intervention First week Second week |
2.3±12.35 2.6±8.5 3.6±5.9 |
2.5±11.67 2.48±10.37 3.2±8.6 |
F=36.2 P<0.0001 |
Table 2: Frequency Distribution of the Changes in Wound Surface Area, Amount of Drainage, Tissue Type, Push Scores over Time in Two Groups of Intervention and Control
|
|
|
First week in comparison to pre-intervention |
Second week in comparison to pre-intervention |
Second week in comparison to first week |
|
Group |
Variable |
Number Percentage |
Number Percentage |
Number Percentage |
|
Intervention |
Wound surface area
Decrease No change Increase |
37 92.5 3 7.5 0 0 |
38 95 2 5 0 0 |
31 77.5 8 20 1 2.5 |
|
Control |
Wound surface area
Decrease No change Increase |
25 62.5 12 30 3 7.5 |
31 77.5 8 20 1 2.5 |
28 70 9 22.5 3 7.5 |
|
Intervention |
Amount of drainage
Decrease No change Increase |
37 92.5
3 7.5 - - |
3 97
1 2.5 - - |
5 12.5
35 87.5 - - |
|
Control |
Amount of drainage
Decrease No change Increase |
12 30
28 70 - - |
31 77.5
9 22.5 - - |
22 55
17 42.5 1 2.5 |
|
Intervention |
Tissue stage
Decrease No change Increase |
31 77.5 9 22.5 - - |
35 87.5 5 12.5 - - |
21 52.5 19 47.5 - - |
|
Control |
Tissue stage
Decrease No change Increase |
16 40 22 55 2 5 |
24 60 16 40 - - |
16 40 23 57.5 1 2.5 |
|
Intervention |
Push scores
Decrease No change Increase |
39 97.5 1 2.5 - - |
40 100 - - - - |
35 87.5 4 10 - 2.5 |
|
Control |
Push scores
Decrease No change Increase |
31 77.5 8 20 1 2.5 |
37 92.5 2 5 1 2.5 |
37 92.5 1 2.5 2 5 |
Table 3: Mean and Percentage of Reduction in Wound Surface Area, Amount of Drainage, Tissue Type, Push Scores over Time in Two Groups of Intervention and Control
|
Variable |
Group |
First week in comparison to pre-intervention |
Second week in comparison to pre-intervention |
Second week in comparison to first week |
|||
|
Mean reduction |
Percentage reduction |
Mean reduction |
Percentage reduction |
Mean reduction |
Percentage reduction |
||
|
Wound surface area |
Control |
0.55 |
8 |
1.37 |
20 |
0.82 |
13 |
|
Intervention |
1.67 |
22 |
3.60 |
49 |
1.93 |
34 |
|
|
Drainage |
Control |
0.35 |
14 |
0.87 |
36 |
0.52 |
25 |
|
Intervention |
1.22 |
50 |
1.35 |
55 |
0.12 |
10 |
|
|
Tissue stage |
Control |
0.4 |
16 |
0.82 |
34 |
0.42 |
21 |
|
Intervention |
0.39 |
35 |
1.5 |
57 |
0.57 |
34 |
|
|
Push |
Control |
1.3 |
11 |
1.07 |
26 |
1.77 |
17 |
|
Intervention |
3.8 |
31 |
6.45 |
52 |
2.62 |
30 |
|
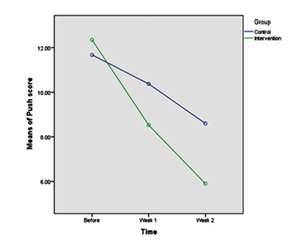
Fig. 1: Comparison of Wound Healing Process by Adding Local Oxygen to Conventional Treatments
DISCUSSION
The aim of this study was to investigate the effects of local oxygen on pressure ulcer in children admitted to the TICU.
The present study results showed that the number of patients with complete wound heal was significantly reduced in the local oxygen therapy group compared to the control group (P<0.0001). Oxygen can reduce the wound surface area, drainage, and formation of granulation tissue in stage 3 pressure ulcers in the patients with severe traumatic injury but with no history of systematic disease. No wound drainage was observed by the end of the first week and the wound surface area was reduced by 95% in the intervention group and by 77.5% in the control group; the degree of ulcer was decreased by 87.5% in the intervention group and by 60% in the control group; moreover, the wound surface area was reduced by 49 % in the oxygen therapy group and by 20% in conventional treatment group. The amount of drainage was decreased by 55% in the oxygen therapy group and in the conventional treatment group, it had 36% decrease. The wound healing was occurred by 57% in the oxygen therapy group and the wound surface area was decreased by 35% in the conventional treatment group.
The effects of oxygen on wound healing have been investigated in many studies. Oxygen is the main factor in the angiogenesis process in wound repair. Angiogenesis results in the saturation of blood vessels and provides a proper environment for wound healing; moreover, macrophages and fibroblasts at the wound site are not useful without oxygen [9]. Oxygen stimulates collagen synthesis, the production of proline and lysine, and cell maturation in the wound. Furthermore, oxygen produces endothelial growth factor, facilitates wound contraction, and stimulates the differentiation of fibroblast to myosyte cells, which increase the formation of granulation tissue. In this case, collagen sedimentation and angiogenesis are increased, and the bacterial proliferation is mostly limited in this condition. Earlier, the effect of hyperbaric oxygen on the healing of pressure ulcers was proven; however, due to the high costs of the tool and the limitation in its use, such as the inaccessibility of the ICU patients to the therapist, using available tools that are inexpensive, we decided to find a way to make use of the effects of oxygen on healing the pressure ulcer. In the method we applied, a sterile coating (urine bag) is placed on the wound since due to transparency, the wound will be visible. The oxygen flow is also checked, the three sides of the urine bag are fixed with an adhesive tape, the urine bag on its loose end, using an oxygen connector, is connected to manometer containing distilled water.
In a study by Agrawall et al. (2015), a method was introduced for using the local oxygen therapy with inexpensive tools, they showed that this method contributes to healing of large and deep wounds which is consistent with our study [8]. In Brent et al. (2006) study, it was showed that the use of local oxygen does not have a positive effect on the healing of diabetic wounds. The discrepancy between the results of the mentioned study and our study is probably due to the study population because our patients had no history of systematic diseases and in Brent’s study, the patients had diabetes mellitus which impedes wound healing [21]. In a study by Landahell et al. (2010), they showed that using local oxygen, the wound surface area was decreased which is consistent with our results [22]. In a study by Sultan et al. (2009), on venous ulcers, two methods of the local oxygen therapy were compared with the conventional method (dressing), and it was concluded that local oxygen reduced the wound surface area, and the drainage, resulted in the formation of granulation tissue, and prevented the relapse of stage 3 pressure ulcer in the patients with traumatic brain injury who had no history of systematic diseases which is consistent with our study results [19]. In a study by Enmut et al. (2017), they showed that hyperbaric oxygen accelerates the healing process of radiation-induced necrotic ulcers, which indicates the positive effects of local oxygen on wound healing which is consistent with our results [23]. In another study by Chang et al. (2016), they showed that the use of hyperbaric oxygen in the patients with burns improves the controlling process in sepsis [24]. In a study by Wu et al. (2012), they showed that oxygen reduced the wound surface area and the amount of drainage, and improved the type of tissue; moreover, the most significant effect was on the reduction of drainage, which is consistent with our study [25]. In a study by Row et al. (2016) on the patients with chronic ulcers, they observed that oxygen causes wound healing, so that oxygen levels are the most effective means to maintain oxygen balance at different stages of wound healing [6]. In a study conducted by Crank et al. (2004) on the patients with chronic ulcers, they showed that local oxygen reduced the wound surface area without side effects, which is consistent with the our study results [26]. In a study by Azimian et al. (2009), they showed that local oxygen reduced the wound surface area, and the amount of drainage, and improved tissue type; its effect on the reduction of drainage was ranked the highest, flowed by the reduction of the wound surface area and improved tissue type and as it is obvious the result of this study is consistent with our study results [27]. In a study conducted by Mehrabani et al. (2012) on the patients with pressure ulcers, they showed that honey reduced pain which is consistent with our study results [28]. The positive effects of hyperbaric oxygen has already been shown; however, due to the lack of appropriate condition for hyperbaric oxygen, its high costs, lack of hyperbaric oxygen chamber, its side effects, and the limitation of its use in ICU patients due to monitoring and ventilator apparatus in many treatment centers, we decided to take measures and provide conditions for oxygen therapy of the wounds.
Limitations
In the present study, all the patients were children with severe traumatic brain injury but without history of systematic disease; and it is possible that transdermal oxygen therapy via the mentioned method would lead to different results with another population or another sample size.
CONCLUSION
Given the study results, the administration of 10 liter per minute for 20 minutes through sterile coating can have positive effects on the healing of stage 3 ulcer in children with a low level of consciousness but with no history of systematic disease. As we observed, oxygen significantly reduces the wound surface area, and the drainage, and forms granulation tissue in this kind of ulcers. It is recommended that the present study be replicated with populations other than children with traumatic brain injury and with patients with systematic disease. Due to the availability and low cost of oxygen in healing the pressure ulcers, the oxygen use in the ulcers treatment of this kind is recommended.
ACKNOWLEDGEMENT
The authors of this article announce their appreciation and thankfulness to Ms. Gholi Nasab, master of Intensive Care Nursing, to head nurses, and to personnel of TICU in Kerman Shahid Bahonar Hospital for their cooperation.
REFERENCES