



|
Dietary Inclusion of Aegle Marmelos Fruits in Wistar Rats Saket Singh Chandel1*, Pushpendra Patel2, Vandana Janghel3, Ram Kumar Sahu4 |
|
1 Department of Pharmacology, Dr. C.V. Raman Institute of Pharmacy, Dr. C.V. Raman University, Bilaspur, Chhattisgarh, India. 2 Department of Pharmacology, Siddhi Vinayaka Institute of Technology & Sciences, Bilaspur, Chhattisgarh, India. 3 Department of Pharmacognosy, Siddhi Vinayaka Institute of Technology & Sciences, Bilaspur, Chhattisgarh, India. 4 Department of Pharmaceutical Sciences, Assam University, Silchar, Assam, India. |
ABSTRACT
Background: The favorer healthful plant Aegle marmelos is historically employed in India. There is no study on the effect of this plant on nephrotoxicity. Objectives: The goal of this study was to investigate the modulatory effects of dietary inclusion of Aegle marmelos against Cisplatin-induced nephrotoxicity in Wistar albino rats. Materials and Methods: Wistar albino rats of clusters I and II were fed basal diets whereas clusters III and IV were administered diets including 2% and 4% Aegle marmelos respectively for 7 days before Cisplatin administration. Cisplatin was administered to rats for 7 days leading to a diminution in the natural processes of the antioxidant activities in the rat. The kidney homogenates were investigated for superoxide dismutase (SOD), Catalase (CAT) and Glutathione (GSH), and activity lipid peroxidation (LPO). The activity of SOD was determined by the Nitro blue tetrazolium (NBT) reduction process. Results: After administration of diets containing Aegle marmelos fruit, a substantial decline in the increased Lipid peroxidation and superoxide dismutase, Glutathione and Catalase concentration was noted. Conclusions: In conclusion, the nephroprotective activity of Aegle marmelos can be due to the inhibitory influence on these plant components as proved by increasing the activity of inhibitory enzymes and considerable modification in the physiological parameters.
Key Words: Aegle marmelos; Antioxidant effect; Cisplatin; Dietary Inclusion; Nephrotoxicity.
INTRODUCTION
The common sources of acute renal injury like Glomerular, interstitial and vascular diseases are synthetic drugs. The damage may be acute and reversible, or chronic and can lead to end-stage renal failure. The participation of the kidney due to drug abuse is either ascribed to their elimination through the kidney or a direct nephrotoxic effect [1]. Cisplatin is mostly prescribed in the chemotherapy of cancer patients, and it is associated with highly toxic properties. Hence, it is required to search novel formulations that can inhibit the toxic properties of cisplatin [2, 3].
Nature has endowed us with a great diversity of medically useful plants [4, 5]. Medicinal plants have healing characteristics and therapeutic values because of the existence of different complex phytochemical compounds [6-8]. This traditional medicine is assumed to have greater importance because of being very effective, more reliable, locally available, and no side effects.
The global market for herbal medicines currently stands at over US $60 billion per year and is growing gradually. Indian ancient traditional system that has been recognized for almost 5000 years. It includes food and herbal remedies while emphasizing the organization and brings in sickness prevention and management [9, 10]. Flora and their secondary metabolite components have an expanded history of usage in contemporary medicine and certain systems of established medical specialty and are the sources of significant drugs. The chemical ingredients present in them have a physiological role in plant and so they are considered to have better compatibility with the human body [11].
The medicinal plants are used to cure several diseases. It is safe compared to synthetic drugs. The leaves, fruits, stems, blossoms, etc. are utilized as a medicine. Further, herbal medicine should be prescribed by trained professionals known as herbalists or botanists [12, 13].
Cisplatin is utilized for the treatment of various human malignancies. Cisplatin induces the generation of reactive oxygen species (ROS) and adversely influences the standard biological functions. ROS interrupts the DNA and results in cell growth. Several DNA damages are mediated by metal-induced free radicals; while in total, metals can prevent the repair of DNA. Several mechanisms lead to patchy up inhibition: (a) directly by free radical, substitution of zinc in Zn-finger domains; or (b) indirectly by turning down the intensity of decreased glutathione. These capacities moderately explain the absurdity that ROS may be carcinogenic, but furthermore useful to treat cancer [14-17].
These diverse effects might take place owing to different concentrations and time of exposure. Metal concentrations more than precise physiological levels are toxic, probably by generating free radicals. Habitually, each cell has equilibrium between free radicals and antioxidants. While ROS might devastate the reduction capacities of the cell, they persuade lipid peroxidation, calcium homeostasis, depletion of the sulfhydryl groups, DNA damage and signal transduction pathways. So, it leads to aging and/or cancer. Even if recent facts underline the importance of Cisplatin-induced reactive oxygen species in vitro, comparable data are still not available for primary tumor tissue. Some reports reveal that Cisplatin induces ROS formation in vivo, which is authentic for the dangerous side effects of Cisplatin therapy, including hepatotoxicity, nephrotoxicity, etc. which is a twist, are cut by the addition of antioxidants. However, accepting the expression of pro-apoptotic proteins and anti-apoptotic proteins and their connection to the redox system in the cell, as considerably as the context of ROS production upon Cisplatin treatment, will provide valuable insight into new approaches for the prevention of Cisplatin side effects devoid of affecting its efficiency [18-21].
Cisplatin is proclaimed for nephrotoxicity property. It has been noted that the utilization of Cisplatin for inflammatory pathogenesis with T lymphocytes results in Cisplatin nephrotoxicity. In the cancer patient, Cisplatin preempts acute renal failure and down-regulation of hepatic cytochrome P450 (P450) in an isozyme selective manner. The inhibitions of these enzymes are due to the fundamental interaction through sulfhydryl groups [21].
The nephroprotective agents are utilized for the protection of the kidney against nephrotoxicity. Traditionally, several plants are utilized for the treatment of kidney failure or dysfunction. The different phytoconstituents namely flavonoids, coumarins, glycosides, and polyphenols, etc. present in plants produce protection to kidney [22]. Aegle marmelos is a regularly utilized food, and their medicinal characteristics have been well recognized since time immemorial. Aegle marmelos is a good source of compounds with a positive impact on human health and those compounds include flavonoids. The findings have shown that Aegle marmelos have profound beneficial health effects including antibacterial, anticarcinogenic, and anti-atherosclerotic properties, and antioxidant activity, etc. In view of its diverse spectrum of pharmacological properties, it is worthwhile to investigate and establish the hepatoprotective and nephroprotective potential of dietary containing Aegle marmelos against gentamicin and Cisplatin-induced hepatotoxicity and nephrotoxicity in rats [23, 24].
MATERIALS AND METHODS
Plant materials
The fruits of Aegle marmelos were collected from the tribal belt during October 2015. The selection of the fruit of the plant was according to the data gathered from the tribal people. Initially, the plant was identified by their vernacular names through consultations with the local citizenry. Consequently, the collected plants were identified and authenticated by the scientist; accordingly, the voucher specimen (AML-1) was deposited in the herbarium in the Institute.
Experimental animals
Wistar albino rats selected for the study were 105–200 gm in weight. The experimental animals received from an animal house verified by the Institutional Animal Ethical Committee (IAEC Registration no- CIP/IAEC/2016-17/080). The IAEC is registered under CPCSEA, New Delhi with the registration number 1321/PO/ReBi/S/10/CPCSEA; dated 22/10/2014. The experimental animals were maintained in the arrangement of 12 h light/dark cycle and the temperature maintained at 25℃ with unrestricted access to food and water ad libitum [23, 24].
Preparation of Dietary inclusion
The basal diet (50% skimmed milk, 36% corn starch, 10% groundnut oil and 4% mineral and vitamin premix) was prepared and fed to normal and control group animals. The basal diet was supplemented with 2% w/w and 4% w/w powdered fruit pulp of Aegle marmelos respectively, and fed to normal and nephrotoxicity-induced animals [23, 24].
Protocol for Grouping of animals
All the animals were separated into five groups; each group consisted of 6 animals and they experienced the treatment as follows:
Group I: Normal and received basal diet
Group II: Received basal diet + 4% w/w Aegle marmelos fruit
Group III: Single administration of Cisplatin (6 mg/kg i.p.) + Control group received Basal diet for 7 days
Group IV: Single administration of Cisplatin (6 milligram/kg i.p.) + 2% w/w Aegle marmelos for 7 days
Group V: Single administration of Cisplatin (6 milligram/kg i.p.) + 4% w/w Aegle marmelos for 7 days
The protocols were ended after 7 days. The animals were decapitated by cervical dislocation. The blood was withdrawn from animals by direct heart puncture and maintained in bottles containing EDTA. The plasma was separated from blood by centrifuging at 3000 r/min for 10 min. Besides, the plasma was utilized for the determination of different biochemical parameters. Correspondingly, the kidneys were isolated, rinsed in cold saline and homogenized in phosphate buffer (pH 6.9). The clear supernatant was obtained after centrifuging the homogenates at 7500 r/min for 10 min. The in vivo antioxidant activity was judged from the clear supernatant.
Biochemical parameters
The uric acid, urea, creatinine, Blood Urea Nitrogen (BUN) and total protein were determined in serum using kits. The body weight, water volume, water pH and kidney weight were assessed in the assessment of kidney function test [25-29].
Analysis of antioxidant enzymes of kidney tissue
The antioxidant actions in the rat kidney homogenate were determined for superoxide dismutase (SOD), Catalase (CAT) and Glutathione (GSH) and activity lipid peroxidation (LPO). The activity of SOD was investigated by the Nitro blue tetrazolium (NBT) reduction process. The H2O2 technique was utilized for the investigation of CAT activity. The substrate 5, 5'- Dithio. bi's (2 – nitrobenzoic acid) was applied for the estimation of the GSH level. The lipid peroxidation level was calculated by measuring the concentrations of malondialdehyde [30-32].
Histopathology of kidney
The kidney was separated from the experimental animals of each group and washed with the normal saline. The isolated organs were fixed at 10% buffered neutral formalin. Afterward, it was processed for paraffin implanting and the microtome technique was followed for sectioning organ. The sections obtained were processed in the alcohol‐xylene series and were stained with alum-hematoxylin and eosin. The stained sections were inspected under a microscope for the assessment of histopathological changes in kidney [23, 24].
Data analysis
Outcomes were analyzed using a one-way analysis of variation (ANOVA) followed by Dennett’s test using SPSS software, Graph Pad Prism; version 5.03. Values were expressed as mean ± SEM and p <0.05 was taken as statistically significant.
RESULTS
Cisplatin is one of the most potent chemotherapy drugs widely utilized for cancer treatment and associated with the adverse impacts that produce harmful effects on vital organs. The kidney is mostly affected by the administration of Cisplatin [33-35]. The untoward effects of Cisplatin are due to the production of oxidative stress in the cellular arrangement. The medicinal plants are a fertile source of several flavonoids and polyphenol along with various phytoconstituents and used for the ascendancy of free radicals created by oxidative stress. Adjuvant therapy is the best method to minimize the side effects on the kidney during the administration of Cisplatin. It has been found that most of the work has been performed on the plant extracts, and very little research has been carried out on the medicinal features of dietary supplement. The medicated dietary supplement is the best alternative herbal preparation to put back the crude extracts which are presently in employment. It is more convenient for administration by patients compared to crude extract [36-38].
Aegle marmelos is a widely utilized food, and their medical characteristics have been well determined since time immemorial. It is reported that flavonoids and phenols possess significant chemoprotective potential [39, 40]. Flavonoids are widely spread in the Aegle marmelos fruit and exhibited various pharmacological activities including nephroprotective activity. In the prospect of the various spectrums of pharmacological properties A. marmelos, it was worthwhile to investigate and establish the nephroprotective potential of dietary containing A. marmelos against Cisplatin-induced nephrotoxicity in Wistar rats.
The nephroprotective activity of A. marmelos dietary supplement against Cisplatin-induced nephrotoxicity
The nephroprotective activity was assessed by determining the levels of uric acid, serum urea, creatinine, BUN and total proteins of normal, Cisplatin and dietary supplement treated animals. The significant increase in the level of urea, creatinine, uric acid, BUN and total proteins in Cisplatin-treated animals compared to normal rats indicates the kidney dysfunction of animals (Table 1 & Fig 1). The animals treated with diets containing 2% w/w and 4% w/w Aegle marmelos significantly decreased the urea, uric acid, creatinine, BUN and total proteins level. The serum level of animals received diets containing only 4% w/w Aegle marmelos (Group II) were normal, and it
reveals that kidney functions were not influenced.
Table 1: Effect of diets supplemented with Aegle marmelos on Kidney function test in animals treated with Cisplatin
|
Group No. |
Treatment |
Urea (mg/dl) |
Uric acid (mg/dl) |
Creatinine (mg/dl) |
BUN (mg/dl) |
Total protein (gm/dl) |
|
I |
Normal rats (Basal diets) |
35.15±0.53 |
1.22±0.45 |
0.51±1.02 |
21.45±5.32 |
5.84±0.89 |
|
II |
Aegle marmelos (4% w/w) + Normal rats |
34.28±0.14 |
1.29±0.93 |
0.45±2.17 |
25.62±3.72 |
5.67±1.24 |
|
III |
Control rats (Cisplatin 6 mg/kg + Basal diets) |
143.82±0.81* |
4.92±0.28* |
1.75±0.57* |
98.17±1.47* |
14.32±2.14* |
|
IV |
Aegle marmelos (2%) + Cisplatin (6 mg/kg) |
95.58±0.21a |
2.75±0.73a |
1.28±1.28a |
65.53±3.05 |
9.63±1.51 |
|
V |
Aegle marmelos (4%) + Cisplatin (6 mg/kg) |
42.26±0.62a |
1.63±0.37a |
0.63±1.63a |
41.26±2.41 a |
5.42±1.95a |
Values are expressed as mean ± SEM, n = 6 in each group. *P<0.05 when compared with normal Group I and Aegle marmelos (4% w/w) Group II, aP<0.05 when compared with Cisplatin (5 mg/kg) treated Group III considered as statistically significant
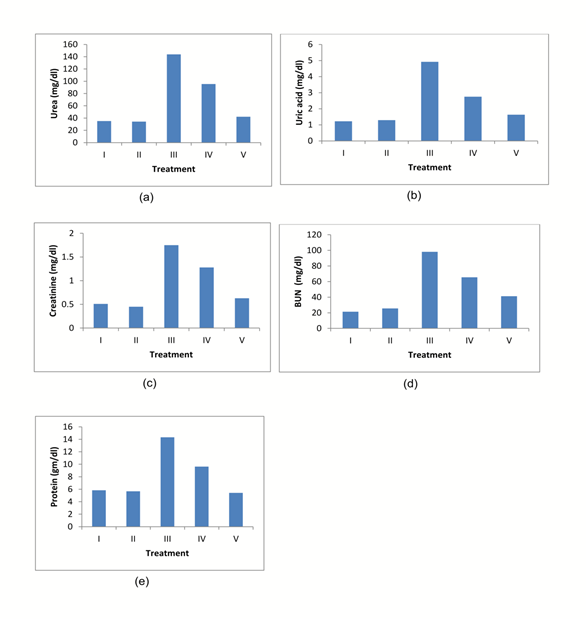
Fig.1. Levels of serum: (a) Urea, (b) Uric acid, (c) Creatinine, (d) BUN, (e) Proteins.
Effect of the dietary supplement on body weight, urine volume, urine pH and kidney weight
The body weight and urine volume of Cisplatin treated animals displayed a significant reduction compared to normal animals (Table 2 & Fig 2). The animals treated with diets containing 4% w/w Aegle marmelos significantly enhanced the body weight and urine volume compared to Cisplatin-treated animals. However, the utilization of diets containing 2% w/w Aegle marmelos indicated no significant increase in body weight and urine volume (Table 2 & Fig 2). The administration of Cisplatin significantly increased urine pH and kidney weight. However, the administration of a diet containing 4% w/w Aegle marmelos significantly decreased urine pH and kidney weight and brought near to normal levels. But the animals fed with a diet incorporating 2% w/w Aegle marmelos failed to significantly decrease urine pH and kidney weight (Table 2).
Table 2: Effect of diets supplemented with Aegle marmelos on Bodyweight, Urine volume, Urine pH, and Kidney weight in rats
|
Group No. |
Treatment |
Body weight (gm) |
Urine volume (ml) |
Urine pH |
Kidney weight (gm) |
|
I |
Normal rats (Basal diets) |
192.14±1.52 |
28.33±0.92 |
5.12±0.32 |
1.18±0.57 |
|
II |
Aegle marmelos (4% w/w) + Normal rats |
186.32±3.14 |
30.18±1.06 |
5.01±0.72 |
1.05±0.81 |
|
III |
Control rats (Cisplatin 6 mg/kg + Basal diets) |
135.65±2.31* |
13.57±0.47a |
8.74±0.73* |
2.32±0.68* |
|
IV |
Aegle marmelos (2%) + Cisplatin (6 mg/kg) |
168.43±1.82 |
20.43±1.02 |
7.12±0.49 |
1.56±0.71 |
|
V |
Aegle marmelos (4%) + Cisplatin (6 mg/kg) |
186.24±2.63a |
24.72±0.84a |
6.05±0.54a |
1.30±0.48a |
Values are expressed as mean ± SEM, n = 6 in each group. *P<0.05 when compared with normal Group I and Aegle marmelos (4% w/w) Group II, aP<0.05 when compared with Cisplatin (5 mg/kg) treated Group III considered as statistically significant.
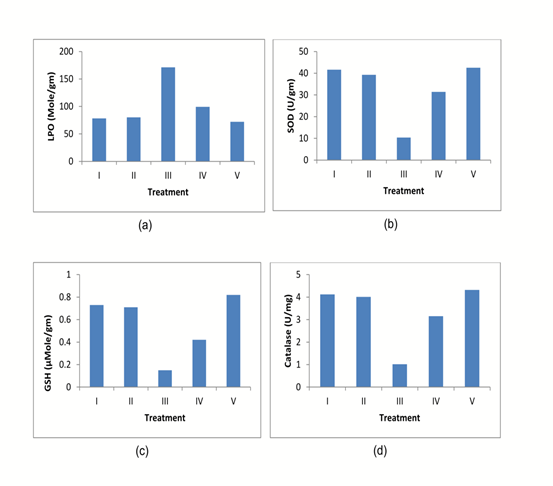
Fig.2. Measurement of: (a) Bodyweight, (b) Urine volume, (c) Urine pH, (d) Kidney weight.
Effects of a dietary supplement on renal antioxidant
The in vivo antioxidant activity of diets containing Aegle marmelos was evaluated. The administration of Cisplatin significantly reduces the LPO, SOD, CAT, and GSH with a marked increase in MDA compared to normal group animals (Table 3 & Fig 3). The animals treated with diets containing 2% w/w and 4% w/w Aegle marmelos significantly increased the LPO, SOD, CAT, and GSH compared to Cisplatin treated animals. Consequently, the MDA level of animals was significantly decreased after administration of diets containing 2% w/w and 4% w/w Aegle marmelos (Table 3).
Table 3: Effect of diets supplemented with fruits of Aegle marmelos on oxidative stress induced by Cisplatin in the kidney of experimental animals
|
Group No. |
Treatment |
Enzymes involved in oxidative stress in the kidney |
|||
|
LPO (Mole/gm) |
SOD (U/gm) |
GSH (μmole/gm) |
Catalase (U/mg) |
||
|
I |
Normal rats (Basal diets) |
78.25±1.53 |
41.63±2.14 |
0.73±0.62 |
4.12±0.95 |
|
II |
Aegle marmelos (4%) + Normal rats |
80.11±2.61 |
39.24±1.57 |
0.71±0.18 |
4.01±0.72 |
|
III |
Control rats (Cisplatin 6 mg/kg + Basal diets) |
171.24±2.19* |
10.38±0.68* |
0.15±0.28* |
1.02±0.54* |
|
IV |
Aegle marmelos (2%) + Cisplatin (6 mg/kg) |
99.34±3.52a |
31.41±1.14a |
0.42±0.83a |
3.15±0.73a |
|
V |
Aegle marmelos (4%) + Cisplatin (6 mg/kg) |
72.15±2.63a |
42.51±3.42a |
0.82±0.41a |
4.32±0.69a |
Values are expressed as mean ± SEM, n = 6 in each group. *P<0.05 when compared with normal Group I and Aegle marmelos (4% w/w) Group II, aP<0.05 when compared with Cisplatin (5 mg/kg) treated Group III considered as statistically significant
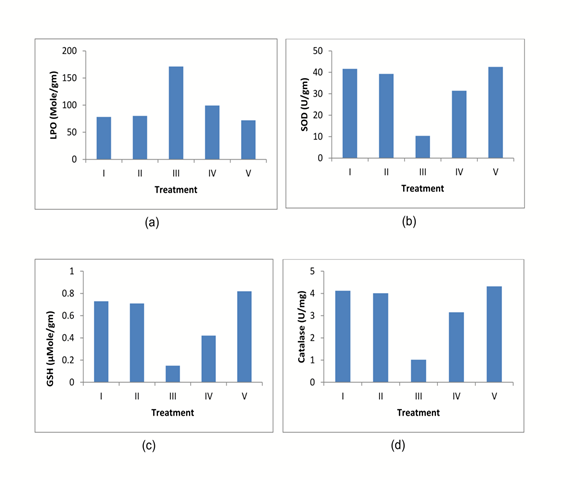
Fig.3. Antioxidant activity evaluation: (a) LPO, (b) SOD, (c) GSH, (d) Catalase.
Histopathology studies of kidney
In basal diet and Aegle marmelos (4%), treated animals showed the normal architecture of the kidney with normal glomeruli, urinary space, Bowman's capsule, podocytes, convoluted tubules, distal tubules and all other structures (Fig 4 & 5). The administration of Cisplatin to animals leads to marked necrosis in proximal tubules and degeneration of the tubular epithelial cells (Fig 6) indicating mild to moderate tubular necrosis in kidneys. Consequently, inflammatory infiltration was observed in Cisplatin-treated animals. The pretreatment with diets containing 2% w/w and 4% w/w Aegle marmelos revealed a protective effect by preserving cellular structure with no sign of inflammation at both doses (Fig 7 & 8).
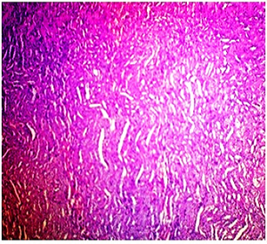
Fig.4. Microscopical photograph of kidney section of normal rats (Group-I) showing histology H & E staining (100x) and normal size of glomeruli with normal tubules
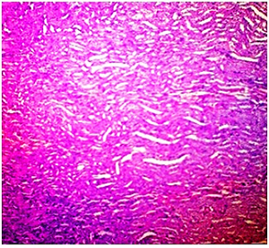
Fig.5. Microscopical photograph of kidney section of normal rats (Group-II) showing histology H & E staining (100x) and normal size of glomeruli with normal tubules.
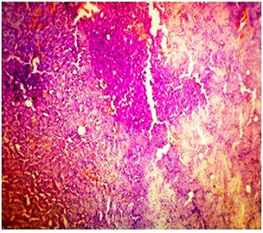
Fig.6. Microscopical photograph of kidney section of Cisplatin treated rats (Group - III) showing Tubular necrosis, tubular dilation and extensive epithelial vacuolization in renal tubules.
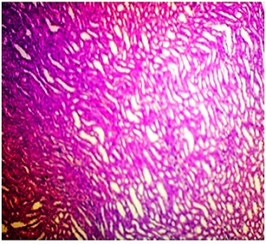
Fig.7. Microscopical photograph of kidney section of Cisplatin + Aegle marmelos (2%) treated rats (Group - IV) showing the reduction in renal cytotoxic cell damage.

Fig.8. Microscopical photograph of kidney section of Cisplatin + Aegle marmelos (4%) treated rats (Group-V) showing recovery from renal cytotoxic cell damage showing the normal size of glomeruli with normal tubules.
DISCUSSION
Cisplatin promotes nephrotoxicity by causing renal phospholipidosis through inhibition of lysosomal hydrolases such as sphingomyelinase and phospholipases in addition to inducing oxidative stress. Drug-induced nephrotoxicity is often related to a marked increase in blood urea, serum creatinine, and acute tubular necrosis. So, these biochemical parameters have been utilized to assess drug-induced nephrotoxicity in animals and man. The present investigation was planned to induce nephrotoxicity against Cisplatin in rats. The renal toxicity was evaluated by measuring the levels of serum urea, creatinine, uric acid, BUN, and total proteins. A significant increase was observed in the level of serum urea, creatinine, uric acid, BUN, and total proteins compared to a normal group of animals [41-43]. The nephrotoxicity was confirmed with varied data of body weight, urine volume, urine pH, and kidney weight in Cisplatin-treated animals. Further, the histological findings confirmed the nephrotoxicity in animals. The kidney function of animals treated with the diets containing Aegle marmelos became normal and no toxic effect on the kidney was observed. The outcomes suggested that diets containing Aegle marmelos at a higher dose were more effective. Thus, diets containing Aegle marmelos establish a nephroprotective effect.
The rats treated with Cisplatin showed a decrease in body weight. The increase in body weight of animals after treatment with diets containing Aegle marmelos was observed. It was proposed that the Cisplatin-induced weight loss might be due to gastrointestinal toxicity and reduced ingestion of food. The dysfunction of the kidney by Cisplatin indicates the side effects associated with this drug. The diets containing Aegle marmelos increased the bodyweight of animals that suggest the prevention of gastrointestinal toxicity. The increase in kidney weight of Cisplatin-treated animals indicates inflammation of the kidney. However, the animals after treatment with diets containing Aegle marmelos exhibited a decrease in kidney weight ascertaining the removal of inflammation from kidney [44-46].
The decrease in antioxidant activity suggests the nephrotoxicity induced by Cisplatin. In the present study, increased serum creatinine and urea were observed in Cisplatin-treated rats may be due to a reduction in glomerular filtration rate. The impairment in kidney function was accompanied by an increase in MDA concentrations in kidney tissue. The above findings were well correlated with the renal histological results. These observations indicated the Cisplatin-induced nephrotoxicity. The animal treated with diets containing Aegle marmelos provides significant protection against Cisplatin-induced nephrotoxicity, with lowering the level of plasma creatinine and blood urea in Cisplatin-treated animals [47].
Decreased concentration of GSH increases the sensitivity of organs to oxidative and chemical injury. The role of GSH, non-protein thiols in the cells, in the formation of conjugates with electrophilic drug metabolites, most often generated by cytochrome P-450-linked monooxygenase, is well-established. Investigations with many models reveal that the metabolism of xenobiotics often generated GSH depletion. Decreased renal GSH can markedly enhance the toxicity of Cisplatin [48]. The depletion of GSH also probably a prime factor that allows lipid peroxidation in the Cisplatin-treated group. The increased GSH activity could partially describe the protection of biomembranes from oxidative attack.
Reduced SOD activity could lead to the initiation and propagation of lipid peroxidation in the Cisplatin-treated group. This may be either because of the loss of copper and zinc, essential for the activity of the enzyme, or because of ROS-induced inactivation of enzyme proteins. The exact mechanism of Cisplatin-induced nephrotoxicity is not well-determined; several investigators have reported that Cisplatin nephrotoxicity is related to LPO in renal tissue. LPO is ascribed to a free radical-mediated chain reaction that damages cell membranes, and prevention of this process by diet is mainly attributed to the potential of scavenger free radicals [49, 50]. In the current investigation, pre-treatment with diets containing Aegle marmelos inhibited the enhancement in LPO induced by Cisplatin in renal tissue, revealing antioxidant activity of Aegle marmelos.
Conflıcts of ınterest
There is no conflict of interest in the present work.
Financial Support:
There is no financial support in the present work.
ACKNOWLEDGMENT
Authors are thankful to the authority of the Columbia Institute of Pharmacy, Tekari, Raipur, Chhattisgarh, India for laboratory facilities and Dr. Sunita Garg, Chief Scientist, Raw Material Herbarium and Museum, Delhi (RHMD), CSIR-NISCAIR, New Delhi, India for identification of the plant and last but not least Prof. (Dr.) Siva Shonkar Nayak, Director cum Principal, Siddhi Vinayaka Institute of Technology & Sciences, Bilaspur, Chhattisgarh, India for his support and guidance.
REFERENCES