



|
Alternative Treatments and Therapies in Central Giant Cell Granuloma: a Narrative Review
Luca Viganò1, Joana Berberi2, Francesco Bruno3, Alessandra Caggiula3, Matteo Di Loreto3, Matteo Pettinicchio3, Alex Vendrame3, Cinzia Casu4* |
|
1 Department of Radiology, San Paolo Dental Building, University of Milan, Italy. 2 IRCCS Ca’ Granda Foundation General Hospital University of Milan, Italy. 3 San Paolo Dental Building, University of Milan, Italy. 4 DDS, Private Dental Practice, Cagliari, Italy. |
ABSTRACT
The Central Giant Cell Granuloma is an uncommon lesion, accounting for less than 7% of all benign jaw lesions. In 1953, Jaffe was the first to describe these lesions as a giant cell reparative granuloma of the jawbones, and in 1971, thanks to Pindborg and Kramer, it was included in the current nomenclature. The etiology of CGCG is unknown, there is also a peripheral type that some authors consider the most common in maxillary bones. WHO defines CGCG as an intraosseous lesion consisting of cellular fibrous tissue that contains multiple foci of hemorrhage, aggregations of multinucleated giant cells, and some trabeculae of woven bone. Several pharmacological treatments have been proposed as an alternative to surgery. We have analyzed intralesional injections of corticosteroids, treatment with animal or human calcitonin, alfa-interferon therapy, use of monoclonal antibodies (denosumab and imatinib) and bisphosphonates. The aim of this study is to analyze and discuss all those therapeutic possibilities, in order to understand what is already known and what needs to be further investigated.
Key Words: central giant cell granuloma, benign jaw lesions, pharmacological treatment for CGCG, bone lesions.
INTRODUCTION
Oral health [1] is one of the most important concerns in the world [2-4]. Central giant cell granuloma is a benign intraosseous lesion first described by Jaffe. It was hypothesized that the lesion is not a true neoplasm but merely the result of a local reparative reaction [5]. The WHO defines CGCG as an intraosseous lesion consisting of cellular fibrous tissue that contains multiple foci of hemorrhage, aggregations of multinucleated giant cells, and some trabeculae of woven bone [6]. In 2004 epidemiological findings of CGCG in a general population were published. In this report, an incidence of 1.1 per 106 was found for the whole population (1.05 per 106 for males, 1.25 for females). This means that the female predilection is not as large as was earlier assumed (M: F= 1: 2). They very rarely affect the mandibular condyle [7]. A study published in 2018 highlights that these lesions were more prevalent in women than in men, at a 1.56:1 proportion. The mean age of the patients was 25.8 ± 15.3 years (range 0-85). The lesions were more prevalent in the mandible in comparison with the maxilla, but there was no clear prevalence concerning the different regions of the jaws [8]. The origin of this lesion type remains unknown; the lesion may be reactive, a developmental anomaly or a benign neoplasm [9-11].
This lesion usually appears as a painless, slow-growing swelling of the jaw. Sensory disturbance and pain are not common. Displacement of teeth sometimes occurs, leading to malocclusion.
Chuong et al. were the first to differentiate between aggressive and nonaggressive lesions on the basis of signs, symptoms, and histological features. Aggressive lesions are characterized by one or more of the following features: pain, paresthesia, root resorption, rapid growth, cortical perforation, and a high recurrence rate after surgical curettage. Aggressive lesions were also larger and histologically demonstrated a larger fractional surface area occupied by giant-cells. Currently, clinical signs and symptoms and radiological features are the main criteria to differentiate nonaggressive (indolent) from aggressive lesions. According to the same studies, the number and volume of giant-cells checked with other components of the lesion might give a sort of prediction on its clinical behavior [12, 13].
In CGCG, 2 major histological features are diagnosed. There is a highly cellular, fibroblastic stroma with plump, spindle-shaped cells with a high mitotic rate; also vascular density is high. These spindle-shaped cells probably are the proliferating tumor cells, considering that they survive in culture after passing wells and immunohistochemically stain positive for the proliferation marker PCNA.
The multinucleated giant cells are prominent throughout the fibroblastic stroma but are not necessarily abundant. They are usually irregularly distributed and are often located most numerously around areas of hemorrhage. Giant cells of CGCG derived from a subset of mononuclear phagocytes that differentiate into mature giant cells under the influence of RANKL-expressing by the proliferating spindle-shaped (osteoblast-like) stroma cells [14-19].
All therapies are based on these considerations, which support corticosteroids injections, calcitonin, alfa-interferon, monoclonal antibodies, and bisphosphonates use. Nevertheless, surgery is still the most commonly applied treatment for CGCG. However, it brings to an inevitable loss of teeth and could damage irremediably the function of inferior alveolar nerve. Moreover, it can lead to aesthetical and functional defects resulting in highly invasive and disabling. Given all these negative aspects, it is questionable whether this type of therapy is the best for a benign lesion such as CGCG.
MATERIAL AND METHODS
Pubmed research has been made. MeSH terms and keywords were “treatment of central giant cell granuloma” resulting in 1420 articles. Only articles published after 1980 and articles with full text available were included.
Inclusion criteria were:
In the end, we obtained 603 potentially useful articles. We excluded not relevant articles after abstracts observation. We analyzed 127 articles and 71 resulted significant (Figure 1).
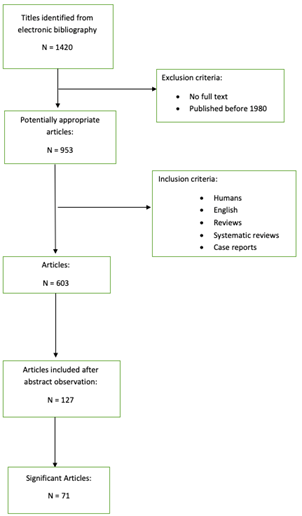
Figure 1. Flowchart of the study design.
DISCUSSION
Surgical Treatment
The most common therapy for CGCG is surgical treatment. The type, location, and size of the lesion can influence intervention. Curettage and enucleation are the most used techniques for small and non-aggressive lesions and are used when periosteum has been infiltrated and there is superficial bone resorption [20]. These conservative and minimally invasive treatments could be accompanied by additional procedures such as cryosurgery and ostectomy and have a recurrence rate at 5 years of 26.3% [21]. Radiotherapy has been also suggested as not invasive treatment, but it can lead to malignant transformation [22].
Aggressive forms have a major probability of relapse compared to non-aggressive lesions. In order to avoid recurrence, en-bloc resection is the main technique used. It is carried out a surgical resection with a 5-mm margin of healthy tissue. This invasive intervention brings an inevitable loss of teeth and could damage irremediably the function of inferior alveolar nerve [23]. Margins of the lesion should be thermally sterilized with a laser cryoprobe before the immediate reconstruction [24] which could be made either with osseointegrated implants or an “overdenture” prosthesis or via autologous iliac crest bone graft [25].
Although surgical therapy results often in the resolution of the lesion and avoids recurrence, it is highly invasive and disabling. Therefore, other therapeutic treatments based on a different hypothesis of the etiology of CGCG have been considered. Moreover, there is the need of finding new strategies to avoid aesthetical and functional defects, such as loss of tooth germs in pediatric patients. In addition to this, reconstruction of large defects could severely affect function and aesthetics, resulting in challenge [23].
Corticosteroids
Injections of corticosteroids were the first not surgical treatment proposed in 1988 by Jacoway et Terry [26]. The resemblance of CGCG to sarcoidosis contributed to developing the initial theory that corticosteroids could inhibit macrophages’ differentiation and consequently their activity [21]. Contemporary studies showed that their use can stop bone resorption through inhibition of lysosomal proteins production by the giant cells, induction of apoptosis in osteoclastic-like cells, and inhibition of transcription factors [27].
In 2010 standardization of the protocol of injection was proposed and the rate of response has been evaluated.
The protocol includes injections of 1mL of solution (20mg/mL triamcinolone hexacetonide diluted in an anesthetic solution of 2% lidocaine/epinephrine 1/200,000 in the proportion of 1 to 1) per centimeter cube of the radioactive lesion, twice a week for six weeks [21]. The scale of evaluation is based on 4 items: 1. Stabilization or recurrence of the lesion with radiographic exam; 2. Absence of symptoms; 3. Increasing of radio-opacity in a radiographic exam that reveals central or peripheral calcification; 4. Increasing of the difficulty of intralesional injection [21].
Advantages of choosing intralesional corticosteroids instead of surgical removal include a lower cost, avoidance of compromising vital structures [28] such as tooth buds, and neurovascular bundles (especially in treating young patients in which short-term risks of pharmacological treatment might be more acceptable then long-term sequelae from surgical intervention) [29]. Furthermore, it has been shown that corticosteroid injections can be combined with surgical treatment when regression has been already obtained [28].
This technique appears to work more successfully in unilocular lesions than multilocular lesions, and this is probably because of the easier access in unilocular lesions, whereas in multilocular lesions some areas can be missed [30].
Intralesional corticosteroid infiltration could be used alone or in combination with other treatments such as bisphosphonates and calcitonin. However, using corticosteroids in combination with bisphosphonates is not laking of risks: in fact, it could result in the development of medication-related osteonecrosis of the jaw [28].
A disadvantage of corticosteroid injections is the discomfort of a long duration of treatment [31].
Application of intralesional steroids has other controversial findings: patients suffering from diabetes, peptic sore, infections, immunocompromised and pregnant individuals are not suitable for this treatment [32].
Although some studies have revealed the efficiency of this treatment on CGCG, about 50% of cases revealed failure: in some cases, corticosteroids injections caused an increase in lesion’s size [33], and in other cases, the lesion ended up more radiopaque than surrounding bony area [34].
Calcitonin
Calcitonin is a peptide hormone produced by Thyroid C-cells. It acts antagonistically to the parathyroid hormone so, in other words, calcitonin causes an increased influx of calcium into the bones. Calcitonin therapy is based on an immunohistochemical study which demonstrates that giant cells in CGCGs are osteoclasts using osteoclast-specific monoclonal antibodies [35]. Moreover, giant cells express calcitonin receptors [36] and therefore this hormone directly inhibits their function. Many studies support the importance of the receptor’s expression in CGCG cells in order to select the best therapy. Vered et al. suggested that the correct decision regarding the appropriate therapeutic method should be based on the immunohistochemical staining scores for glucocorticoid and calcitonin receptors for each lesion. Moreover, they supported the theory according to which CGCG may be a lesion where constitutional cells undergo a phenotypic transformation with consequent alteration in biological behavior [37]. Nogueira et al. found that immunohistochemical staining for glucocorticoid receptors may provide a tool for selecting the therapeutic strategy, while calcitonin’s receptor results were no statistically significant. However, this study included only 18 cases [38]. Despite these considerations, an important issue in treating CGCG with corticosteroids and calcitonin is the “escape phenomenon”. Continuous and long-lasting administration of calcitonin causes a significant decrease in expression of the calcitonin’s receptor gene by an unknown mechanism [39]. Combining calcitonin with steroids, the escape phenomenon is usually attenuated (and also by dis-continued administrations of calcitonin, such as every couple of days instead of daily) [40]. Harris was the first to propose daily subcutaneous injections of calcitonin as an alternative to surgery for aggressive central giant cell granuloma. Because of histological similarities between CGCG and brown tumor of hyperparathyroidism, he supposed the existence of an unidentified parathormone-like hormone that could be the etiology of the lesion. In his study on 4 patients, lesions achieved full remission [41]. Calcitonin’s successful use as therapy in CGCG lesions is also reported by others [40, 42, 43]; however, Kaban et al. [44] reported ongoing growth of a lesion during human calcitonin treatment. In some countries, only salmon calcitonin is available. Even if it is supposed to have a stronger effect than human calcitonin, it is also more immunogenic (antibodies development can limit its effectiveness) [45]. In addition, an in vitro study showed that there is no difference in the effect of human or salmon calcitonin on the inhibition of osteoclastic bone resorption [46]. Only one randomized double-blind placebo-controlled study has been done. 14 patients were treated with intranasally administered salmon calcitonin (200 UI/day) or a placebo daily. Although in half of the patients CGCG lesion showed a reduction, no complete remission was observed. Anyway, due to the limited number of patients, the power of that study is restricted [47]. Other studies, nevertheless, showed that calcitonin nasal spray can be a treatment option for CGCG lesions [48, 49].
The main problem remains calcitonin’s bioavailability, which is 70% in subcutaneous injections and 3% to 25% in a nasal spray.
In summary, using calcitonin as a therapy for CGCGs has several pro: it is far less aggressive than surgery, it does not harm the patient (even if there are some mild side effects) and it can be an option after calcitonin’s receptors laboratory testing. We cannot as well forget, some cons: long-lasting therapy and costs.
Alfa-Interferon
Interferon-alpha is a cytokine with antiviral and anti-angiogenic properties. The mechanism of action of interferon is inhibition of production of angiogenesis factors: vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF) [34].
Thanks to these properties, alpha interferon has been used for various conditions such as treatment of hemangiomas and many other types of malignant vascular tumors that are deprived of the sustenance of new vessels. INF is either produced by recombinant DNA technology or it is purified from cultured human cells [50].
Given the great success of alpha interferon on these tumors, it was thought to try to use this drug also on CGCG [51] which, although not a real proliferative vascular lesion, has mononuclear cells and giant cell agglomerates with a high immunoreactivity for angiogenetic factors that most likely play an important role in the osteoclastogenic process and therefore contribute to the growth of the lesion [52].
The first known case report of treatment of a CGCG with alpha-2a interferon was published in 1999 by Kaban et al [44]. By analyzing the various case-reports in the literature, it is possible to note that when alpha-interferon monotherapy was applied, good results were obtained such as stabilization of the lesion or even a slight regression, but only in one case, it was possible to obtain complete remission [53].
Another aspect to consider is the side effects caused by this therapy. The most common mild effects are nausea, headache, and fever. However, there are some serious side effects such as skin rash, latargia, neuropathy, thrombocytopenia, and increased liver transaminases that occur in 15% of patients [54, 55].
Considering that no remission was obtained and all possible side effects, it was decided to abandon monotherapy and take advantage of the positive effects of alpha interferon combining it with other therapies.
Kaban et al. a few years later from their 1999 article, published another study on 26 cases of CGCG in which conservative surgery (curettage) was applied and subsequently the administration of interferon-alpha of 3,000,000 U / m2 was administered. The aim was avoiding frequent post-operative recurrences and thus preventing the application of more invasive therapies such as en-bloc surgery, obtaining excellent results [56, 57].
Furthermore, in this article it was reported that surgical treatment is the most common choice, but, using alpha-interferon in combination greatly reduced operational morbidity and determined greater control of the lesion if it was very aggressive [58].
In another study, they proposed therapy with a monoclonal antibody called imatinib combined with interferon-alpha. Imatinib causes a reduction of RANK receptors which activation is normally a necessary step to give rise to osteoclasts. These two drugs inhibit two different aspects of osteoclastogenesis and therefore together they could be stronger than alone [59].
Monoclonal Antibodies
A possible target in controlling CGCG is osteoclastogenesis’ proteins. One of these proteins, RANKL, an essential cytokine for osteoclastogenesis, has recently been demonstrated in CGCG [60]. Osteoclast formation involves interaction between stromal cells, which express RANKL, and mononuclear osteoclast precursors expressing RANK. Moreover, RANKL is also a powerful stimulator of osteoclast bone-resorption activity [61]. Because giant cells in CGCG are osteoclasts [37], osteolytic expansion in CGCG can theoretically be controlled by therapeutic agents that inhibit the RANKL/RANK interaction.
These processes are inhibited by osteoprotegerin (OPG), which is a decoy receptor for RANKL, and also by a monoclonal antibody to RANKL like denosumab.
Denosumab
Denosumab is a human monoclonal IgG2 antibody derived from mammalian cell lines and inhibits activation and differentiation of osteoclast-like giant cells and consequently osteolytic damage by binding RANK-ligand [62, 63]. Denosumab was approved by the U.S. Food and Drug Administration (FDA) to treat adults and skeletally mature adolescents with unresectable GCTB or when resection is likely to result in severe morbidity [64, 65].
Neoadjuvant treatment of CGCG with denosumab may cause a reduction in volume or even a re-ossification of the cyst [66, 67] and can effectively downstage tumors to facilitate less morbid surgery or completely avoid the need for resection. However, there is concern about local recurrence post-surgery.
A larger study showed no disease progression in 69% of patients after median 13 months of treatment, and of 100 patients with salvageable GCTB, 74 needed no surgery and 16/26 less morbid surgery than previously scheduled [64]. All patients were treated with denosumab injections 120mg subcutaneously monthly, for sixth months, either as an alternative to surgery or if the disease had recurred after the initial surgery. In all cases, the ossification of CGCG lesions was described, and in some regression. Several responses were confirmed histologically with a repeated biopsy that did not show any residual osteoclast-like giant cells or granular tissue [66, 67].
The main side effects related to treatment are headache and back pain. Using denosumab 120 mg per month could potentially cause osteonecrosis of the jaws. For this reason, in France, ANSM contraindicates using denosumab in children [65].
Imatinib
Imatinib is a protein tyrosine kinase inhibitor used to treat chronic myeloid leukemia (CML) and gastrointestinal stromal tumors by inhibiting bcr-abl and stem cell factor receptor (c-kit) tyrosine kinases, respectively [68, 69]. Imatinib is well tolerated and shows mild to moderate side effects, notably anemia and skin rashes [69]. In a recent article, the effect of imatinib on osteoclasts was examined [70]. The results of this study indicate a dose-dependent decrease in RANK. This finding strongly suggests that imatinib may be an effective anti-osteolytic agent and could, therefore, be useful in the treatment of skeletal diseases involving excessive osteoclast activity, such as CGCG.
In summary, monoclonal antibodies might be a possible efficient alternative therapy even if we need further research. They are the latest generation drugs available and their skills and applications can achieve great results in many fields, provided clinical trials to demonstrate their effectiveness.
Bisphosphonates
Bisphosphonates are widely used to inhibit osteolysis and osteoclasts’ action in conditions such as osteoporosis, Paget’s disease, and bone destruction through metastatic cancer.
Bisphosphonates have a high affinity for hydroxyapatite binding sites on bony surfaces. They deposit readily in areas of high bone turnover, where they are then phagocytosed by osteoclasts. The ability of bisphosphonates to inhibit bone resorption results from directly impairing the function of resorbing osteoclasts and from decreasing osteoclast progenitor development [71]. Landsberg et al. [72] reported three cases of central giant cell lesions treated with bisphosphonates: the first case resulted in a success, with total remission of the lesion; the second showed a reduction of 30% of the lesion; the last case showed stabilization but not regression of the lesion. Chien et al. [73] demonstrated that therapy with Zoledronic acid is a reasonable option for children with CGCG that has relapsed or is refractory to alternative therapies. Moreover, patients well tolerated ZA therapy. In order to provide a sort of clinical protocol, they suggested that short courses of ZA administered monthly may be adequate [73]. Da Silva et al. [74] and De Mendonça et al. [75] tried to treat CGCG with intralesional injections of corticosteroids in association with bisphosphonates. In the first, the combination of alendronate with corticosteroids did not appear to have benefits in treating CGCL (however, it was not a clinical trial with a large sample size, which would be necessary to confirm the advantages of this association) [74]. In the latter, corticosteroid treatment was provided in association with alendronate sodium and calcium carbonate to promote bone formation and achieve a better prognosis: this therapy permitted to avoid surgery and damage to maxillofacial function and aesthetics [75].
The use of intralesional corticosteroids in combination with bisphosphonates also carries risks, such as the development of medication-related osteonecrosis of the jaws after dentoalveolar surgery. Nevertheless, this technique could be better studied in depth.
CONCLUSION
All these therapeutic strategies are promising, but, at this moment, there are no relevant indications regarding their use.
Our review indicates the need for further clinical trials which include laboratory testing, in order to find the best therapy for each lesion. Treatments need to be individualized according to the clinical, microscopic and molecular markers, which are related to the variations in recurrence and aggressiveness of the lesions.
Further research about the inter-relation between pathogenic mechanisms and clinical behavior are essential in order to develop effective combined therapeutic protocols.
Moreover, other studies are requested to find a nonsurgical option that could affect directly the proliferating cells in CGCGs, which are the stromal mononuclear spindle-shaped cells. Theoretically, acquiring control of these cells would give the greatest therapeutic benefit.
REFERENCES