



|
A Comparison of the Efficacy of Dexamethasone and Ketorolac in Preventing Postoperative Pain and Side-effects in Gynecologic Laparoscopic Surgeries: A Double-blind Randomized Clinical Trial |
|
Zahra Asgari(MD)1, Bahareh Meybodi(MD)1*, Masoumeh Nataj Majd(MD)2, Reyhaneh Hosseini(MD)1, Farahnaz Hajibeygi(BSc)1 |
|
|
|
1 Department of Gynecology, School of Medicine, Arash Hospital, Tehran University of Medical Sciences, Tehran, Iran. 2 Department of Anesthesiology, School of Medicine, Arash Hospital, Tehran University of Medical Sciences, Tehran, Iran. |
ABSTRACT
Background: The impact of preoperative administration of dexamethasone and ketorolac on postoperative analgesia and side-effects of laparoscopic surgeries is acknowledged. However, the efficacy of dexamethasone versus ketorolac has not been compared in any earlier investigations, which was the main goal of this study.
Methods: In a randomized, double-blind placebo control study, 114 females who were elected for minor laparoscopic surgeries were randomly allocated into the three study groups: 30 mg preoperative intravenous ketorolac, 8 mg preoperative intravenous dexamethasone, and preoperative placebo (normal saline). Postoperative pain was evaluated at 1h, 6h, 12h, and 24h after the laparoscopy using the Visual Analog Scale (VAS) for pain. Postoperative opioid consumption and the occurrence of nausea and vomiting were also recorded.
Results: Characteristic features of the study groups were similar. In comparison with the placebo group, postoperative pain over time and opioid consumption were significantly lower in the dexamethasone (p<0.001 and p=0.02, respectively) and ketorolac group (p<0.001 and p=0.002, respectively), while the occurrence of nausea and vomiting was significantly lower only in the dexamethasone group (p=0.03 for both). In comparison with the dexamethasone group, early postoperative pain was lower in the ketorolac group (p=0.01), while the incidence of postoperative nausea and vomiting was significantly smaller in the dexamethasone group (p=0.045 and p=0.03). The opioid usage was not significantly different between the dexamethasone and the ketorolac group (p=0.3).
Conclusion: In minor laparoscopic surgeries, preoperative ketorolac is more efficacious for postoperative pain control, and dexamethasone can be used for patients in whom postoperative control of nausea and vomiting is a priority.
Key Words: Laparoscopy, Dexamethasone, Ketorolac, Pain.
Laparoscopy is a minimally invasive surgery that contains several advantages in comparison with open procedures, including earlier regain of bowel function, lower postoperative pain, sooner recovery, and less hospital stay [1]. Although the extent of pain has significantly decreased following the implication of laparoscopy techniques, the postoperative pain and its effect on the recovery of the patients remains a remarkable concern. In addition to the pain at the sites of the incisional and trocar insertion, shoulder and diffuse abdominal pain caused by peritoneal stretching and diaphragmatic irritation could delay the recovery of patients, leading to more hospitalization and increased financial burden on both the patients and healthcare systems [2]. Therefore, effective pain management after the laparoscopic procedure is of critical importance.
Despite numerous studies on pain management after laparoscopy, there is still no consensus on several aspects of this field, including the timing, route, dose, and type of the analgesic administration [3]. Concerning the type of analgesic drugs, a wide range of medications, including Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), opioids, and corticosteroids, are being used for post-laparoscopic pain management [3]. Even so, there is no agreement on the optimal choice of analgesic drugs for pain relief after laparoscopy [3].
Corticosteroids have long been used to decrease inflammatory response and postoperative pain in various surgeries. Dexamethasone, as a widely used corticosteroid, is considered a powerful anti-inflammatory drug that may also prevent postoperative nausea and vomiting. The efficacy of dexamethasone in laparoscopic pain management has been demonstrated in many investigations [4, 5]. Ketorolac is an NSAID that is commonly used for short-term moderate to severe painkilling in various conditions such as post-surgical pain [6]. The efficacy of ketorolac for pain management after laparoscopy has been also investigated in several investigations [7-9].
As far as we are informed, no study has been carried out to compare the analgesic effects of dexamethasone with ketorolac for pain relief after laparoscopy. In this randomized clinical trial study, we aimed to compare the analgesic impacts of a single-dose of preoperative intravenous dexamethasone versus ketorolac in patients who undergo minor gynecologic laparoscopic surgeries.
PATIENTS AND METHODS
This randomized controlled trial was confirmed by the ethics committee of our university under the code of IR.TUMS.Medicine.REC.1398.9412. The protocol of the study was registered in the Iranian Registry of Clinical Trials under the code of IRCT20110530006640N7. Written informed consent was obtained from the patients before their participation in the study. All the procedures were carried out by the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as revised in 2000 (available at http://www.wma.net/e/policy/17-c_e.html).
Between January 2019 and March 2020, women who were elected for minor gynecologic laparoscopic surgeries (diagnostic laparoscopy, cystectomy, salpingectomy, oophorectomy, and tubectomy) were evaluated for the study eligibility criteria. Inclusion criteria were the age of 18-65 years, body mass index <30 kg/m2, and American Society of Anesthesiologists (ASA) grade I or II. Exclusion criteria were allergy to analgesics, anticoagulant consumption during the last four weeks, daily intake of opioids or anti-anxiety medications, fever, or bacterial infection within the last two weeks (Figure 1).
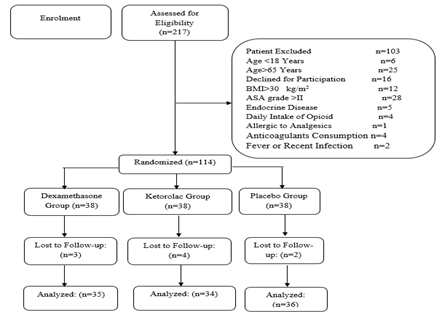
Figure 1: Flow Diagram of the Study Inclusion and Exclusion
Intervention
Patients who met the criteria of the study were randomly assigned to the three study groups. The first group received 30 mg intravenous ketorolac (15 mg/ml, 2 ml). The second group received 8 mg intravenous dexamethasone (4 mg/ml, 2 ml). The third group (placebo group) received 2 ml intravenous normal saline. All the injection was performed 30 min before the laparoscopy. All patients received similar anesthesia (general anesthesia with intubation).
Randomization and Blinding
Randomization was done using a computer-generated list. For this purpose, 114 sealed envelopes were numbered 1-114 according to the randomization list. The randomization codes were kept separate until the study was completed. The randomization ratio was 1: 1, with 38 envelopes allocated to each group distributed in parallel trial design. A nurse that was not involved in the study opened a sealed envelope in a closed medicine room 30–60 min before the operation. The analgesics or placebo were drawn into a 2-ml syringe and intravenously injected into the participants. All solutions were transparent and visually similar. Therefore, both injector and recipients were kept blinded to the group allocation.
Outcome Measures
The primary outcome measure was postoperative overall pain (incisional, visceral, and shoulder pain) that was assessed using the visual analog scale for pain at 1, 6, 12, and 24 hours after the operation. Accordingly, the pain ranged from 0 (no pain) to 10 (most severe pain). The secondary outcome measures were the number of postoperative opioid consumption and the incidence of Postoperative Nausea and Vomiting (PONV) within 24 h after the surgery.
Sample Size Calculation and Statistical Analysis
The sample size was calculated using the Standard Deviation (SD) of 2.23 for VAS provided in the study of Zangoue et al. [10]. According to this SD, an effect size of 1.5, power of 80%, a significance level of 5%, and considering 10% dropouts, many 38 patients were included in each group of study.
SPSS for Windows, version 16 (Chicago, Illinois, USA) was applied for all statistical analyses. Qualitative variables were demonstrated by number and percentage. Quantitative data were presented as mean ± SD. A One-way-ANOVA test was used to compare the characteristic features of the three study groups. Repeated measure Analysis of Variance (ANOVA) was used to compare pain between the groups over time. Qualitative variables were compared with a Chi-square test. The line charts were demonstrated with Microsoft Excel 2010. A p-value of ≤ 0.05 was considered statistically significant.
RESULTS
Demographic Characteristics
One hundred fourteen patients who underwent minor laparoscopic surgery were randomly divided into three study groups. The study populations were females with a mean age of 44.2±11.3 years (Range 23-62). Diagnostic laparoscopy was the most common laparoscopy procedure (n=56, 49.1%) followed by tubectomy (n=24, 21%). No significant difference was observed between the characteristics features of the three study groups (Table 1).
Table 1: Comparison of Characteristic Features between Three Study Groups
|
Variable |
Ketorolac Group (n=38) |
Dexamethasone Group (n=38) |
Placebo Group (n=38) |
P Value |
|
Age (Year) |
43.9±11.2 |
44.1±10.9 |
44.4±11.6 |
0.61 |
|
Body Mass Index (kg/m2) |
25.1±4.1 |
24.8±3.8 |
24.6±3.6 |
0.73 |
|
ASA Type
|
31 (81.6) 7 (18.4) |
33 (86.8) 5 (13.2) |
32 (84.2) 6 (15.6) |
0.54 |
|
Laparoscopy Procedure
|
19 (50) 8 (21) 4 (10.5) 4 (10.5) 3 (8) |
17 (44.6) 10 (26.3) 5 (13.1) 3 (8) 3 (8) |
20 (52.6) 6 (15.8) 5 (13.1) 4 (10.5) 3 (8) |
0.44 |
|
Surgery Duration (min) |
66.2±12.6 |
68.3±14.2 |
65.4±13.1 |
0.39 |
Data are presented as mean±SD or number (%). P<0.05 is considered significant.
Comparison of Outcome Measures between Dexamethasone and Placebo Group
The mean VAS for pain over time was significantly smaller in patients of the dexamethasone group compared to the patients of the placebo group (p<0.001) (Figure 2A). Opioid was administered for 47.2% (17 of 36) of the patients of the placebo group and 20% (7 of 35) of patients of the dexamethasone group (p=0.02). During the 24 h after the laparoscopy, moderate to severe nausea was recorded in 27.7% (10 of 36) of patients of the placebo group and 5.7% (2 of 35) of patients of the dexamethasone group (p=0.03). Vomiting was recorded in 22.2% (8 of 36) of the placebo group and 2.9% (1 of 35) of patients of the dexamethasone group (p=0.03).
Comparison of Outcome Measures between Ketorolac and Placebo Group
The mean VAS for pain over time was significantly smaller in patients of Ketorolac group compared to the patients of the placebo group (p<0.001) (Figure 2B). Opioid was administered for 47.2% (17 of 36) of the patients of the placebo group and 8.8% (3 of 34) of patients of the ketorolac group (p=0.002). During the 24 h after the laparoscopy, moderate to severe nausea was recorded in 27.7% (10 of 36) of patients of the placebo group and 23.5% (8 of 34) of patients of the ketorolac group (p=0.8). Vomiting was recorded in 22.2% (8 of 36) of the placebo group and 20.5% (7 of 34) of patients of the ketorolac group (p=0.9).
Comparison of Outcome Measures between Ketorolac and Dexamethasone Group
The mean VAS for pain over time was significantly smaller in patients of Ketorolac group compared to the patients of the dexamethasone group (p=0.01) (Figure 2C). Opioid was administered for 20% (7 of 35) of the patients of the dexamethasone group and 8.8% (3 of 34) of patients of the ketorolac group (p=0.3). During the 24 h after the laparoscopy, moderate to severe nausea was recorded in 5.7% (2 of 35) of patients of the dexamethasone group and 23.5% (8 of 34) of patients of the ketorolac group (p=0.045). Vomiting was recorded in 2.9% (1 of 35) of the dexamethasone group and 20.5% (7 of 34) of patients of the ketorolac group (p=0.03).
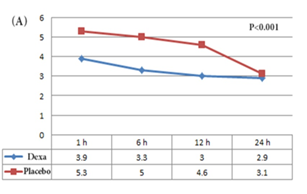
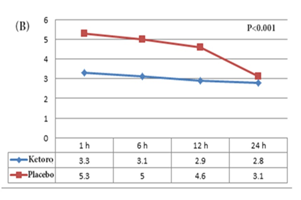
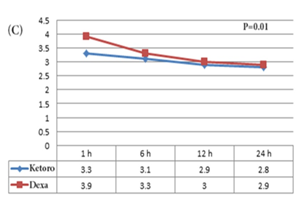
Figure 2: Comparison of VAS for pain of the different time points between (A) the dexamethasone & placebo group; (B) the ketorolac & placebo group; and (C) the ketorolac & dexamethasone group.
DISCUSSION
In the present study, we compared the efficacy of preoperative dexamethasone and ketorolac with each other as well as with placebo on the management of postoperative pain, opioid consumption, nausea, and vomiting frequency after minor laparoscopic procedures. According to our results, both dexamethasone and ketorolac significantly reduced postoperative pain and opioid consumption when compared with the placebo. Dexamethasone, but not ketorolac, significantly reduced the occurrence of postoperative nausea and vomiting when compared with placebo. Dexamethasone's effect on pain control was significantly lower than ketorolac. However, the extent of nausea and vomiting was significantly lower in the dexamethasone group compared to the ketorolac group.
The efficacy of dexamethasone on post-laparoscopic analgesia and side-effects has been investigated in several other investigations. Yamanaga et al., in a retrospective study, evaluated the efficacy of a single perioperative injection of dexamethasone on postoperative nausea, vomiting, and pain level of patients after laparoscopic donor nephrectomy. They categorized patients into three groups: low-dose dexamethasone (4-6 mg, n=70), high-dose dexamethasone (8-14 mg, n=100), and control group (no analgesic, n=110). According to their report, high-dose dexamethasone significantly reduced the occurrence of postoperative nausea and vomiting compared to the control group. Total opioid consumption was also significantly lower in donors who received the higher dose of dexamethasone than in control [11]. We used a high dose of dexamethasone in the present series (8 mg). Similar to the study of Yamanaga et al., the occurrence of nausea and vomiting, as well as opioid consumption, were significantly less in the dexamethasone group than in the control group.
Mohtadi et al., in a prospective study, compared the analgesic impact of a single dose of dexamethasone injection (n=61) with the control group (n=61) on decreasing postoperative pain in laparoscopic cholecystectomy. According to their results, postoperative pain intensity was, and opioid consumption was significantly lower in the dexamethasone group [12]. The same results were observed in the present study.
Bisgaard et al., in a randomized, double-blind placebo-controlled trial evaluated the efficacy of preoperative dexamethasone on the improvement of surgical outcome after laparoscopic cholecystectomy. In this study, 88 patients were randomly allocated to either the intravenous dexamethasone (8 mg) or placebo group. Injections were performed 90 minutes before laparoscopy. According to their results, dexamethasone significantly decreased postoperative
levels of CRP, fatigue, overall pain, incisional pain, and opioid consumption during the first postoperative 24 h. Dexamethasone also significantly reduced nausea and vomiting on the first day after the operation [13]. We also observed a significant effect of preoperative dexamethasone on the reduction of pain and opioid consumption, as well as lowering the incidence of nausea and vomiting compared to the control group.
Efficacy of preoperative dexamethasone on post-laparoscopic pain and side-effects has been investigated in several other investigations, as well. In a systematic study and meta-analysis study, Waldron et al. investigated the effect of perioperative dexamethasone administration on postoperative analgesia and side-effects. According to their analysis, patients who received preoperative dexamethasone reported significantly lower pain during the first 24 h after laparoscopic surgery, but preoperative dexamethasone revealed no significant effect on opioid consumption [4].
The effect of preoperative ketorolac on post-laparoscopy analgesia and side-effects has also been investigated in some studies. Chow et al., in a prospective, double-blind controlled study investigated the impact of preoperative ketorolac administration on postoperative pain intensity in patients undergoing laparoscopic urologic surgery. According to their results, preoperative ketorolac administration significantly reduced the subjective pain perception of pain and narcotic usage compared to the control group [14]. The present study also revealed the significant impact of preoperative ketorolac on decreasing postoperative pain intensity and opioid consumption.
Yeon Hong studied the impact of preoperative ketorolac on the pain intensity in patients who underwent laparoscopic surgery for endometriosis. Fifty patients were randomly assigned to either ketorolac (0.5 mg/kg) or the control group. According to this study, total meperidine dose and VAS for pain were significantly lower in the ketorolac group than in the control group. The occurrences of postoperative side effects, such as nausea, were not significantly different between the groups [8]. Similar to the study of Yeon Hong, postoperative pain was significantly lower in the ketorolac group of the present study. Moreover, no significant difference was seen between the occurrence of postoperative nausea and vomiting between the ketorolac and the control group.
The positive impact of preoperative ketorolac administration on post-laparoscopic pain management has also been demonstrated in other investigations [15, 16]. However, as far as we are informed, no study has been carried out to compare the efficacy of preparative dexamethasone versus preparative ketorolac on the analgesia and side-effects of laparoscopic surgeries. According to our analysis, early postoperative pain intensity was significantly lower in patients who received ketorolac, whereas the occurrence of nausea and vomiting was significantly lower in the dexamethasone group. These findings reveal that preoperative dexamethasone could be regarded as a better option for patients who are at a higher risk of postoperative nausea and vomiting such as patients with a background of motor sickness of migraine [17].
The present study was not without limitations. The main limitation of the study was the heterogeneous etiology of laparoscopy in our patients. Although types of laparoscopic surgery were not significantly different between the study groups, a more homogenous group of patients would better demonstrate the efficacy of ketorolac versus dexamethasone on the improvement of surgical outcomes.
CONCLUSIONS:
In minor gynecologic laparoscopic surgeries, preoperative administration of dexamethasone and ketorolac both reduce the postoperative pain intensity and opioid usage compared to the control group. In comparison with ketorolac, preoperative dexamethasone provides better control of nausea and vomiting and lower control of early postoperative pain. Therefore, dexamethasone is a better choice in patients susceptible to postoperative nausea and vomiting.
ACKNOWLEDGMENTS:
None
Conflict of Interest:
The authors declare no conflict of interest to disclose.
REFERENCES