|
Synthesis and in Vitro Evaluation of Coupled Mercaptobenzimidazole Derivatives Used as a Potent Biological Agent Deepak Kardile *, Mrunal Shirsat |
|
Department of pharmaceutical sciences, Madhav University, Pindwara, Rajasthan, India. |
ABSTRACT
In the present study, coupled mercaptobenzimidazole derivatives were efficiently synthesized, which were further characterized and authenticated by means of TLC, and different spectral data such as IR and 1H NMR. The synthesized compounds DPK2d1 to DPK2d8 were screened for their in vitro antimicrobial and antitubercular activities. The result showed that the titled compounds exhibited potent to moderate antimicrobial activities against Bacillus subtilis, Escherichia coli (antibacterial) and Aspergillus niger (antifungal) as compared to ciprofloxacin and fluconazole, respectively. Compounds DPK2d1, DPK2d2, and DPK2d3 exhibited potent to moderate antitubercular activities against Mycobacterium tuberculosis as compared to pyrazinamide, ciprofloxacin, and streptomycin.
Key Words: Coupled mercaptobenzimidazole, antimicrobial activity, antitubercular activity.
INTRODUCTION
In the 20th century, chemotherapy has revolutionized the treatment of infective diseases since the innovation of antibacterial dyes by Paul Ehrlich and covered the way to a great victory for human health and long life. The development of resistance against currently used antimicrobial drugs led to an invigorated curiosity of the researchers in infective diseases to develop new chemical entities to battle them [1-3]. Patient morbidity, costs of treatment, rates of hospitalization, and use of broad-spectrum agents are remarkably increased by antimicrobial resistance [4-6].
Tuberculosis is a deadly disease usually caused by Mycobacterium tuberculosis. It has killed an estimated one billion people over the last two spans and even remains among the top ten causes of death in the world. According to the 2018 report of WHO, 558,000 people developed rifampicin-resistant (RR TB), multidrug-resistant tuberculosis (MDR-TB) or extensively drug-resistant TB (XDR TB) in the world. Therefore, it is essential to develop rational chemotherapeutic agents to delay the emergence of resistance and, ideally, shorten the duration of therapy of this infection [7-9].
Benzimidazole is a lead molecule for most of the biological agents used in the pharmaceutical industry. It consists of a fused benzene ring with heterocyclic aromatic imidazole. The existence of imidazole creates it resourceful heterocycles with an extensive range of biological activities such as antiulcer (Gastric H+/K+-ATPase inhibitors), antihypertensive, anti-inflammatory, anticonvulsant, analgesic, antiprotozoal, antitrichinellosis, antidiabetic, anti-HIV, antimicrobial, antitubercular, anticancer, antihistaminic, antioxidant, antiviral, antiparasitic agents, diuretic, and DNA binding activities [10-23].
Encouraged by the upstairs findings and in the persistence of our work on coupled mercaptobenzimidazole derivatives, we herein report the synthesis and in vitro evaluation of coupled mercaptobenzimidazole derivatives as a potent biological agent.
MATERIALS AND METHODS:
The chemicals of analytical grade required for the synthesis of coupled mercaptobenzimidazole derivatives were purchased from Sigma-Aldrich and SD fine chemicals (India). Synthesized compounds were determined for their melting points with the help of precision melting point apparatus and were uncorrected. Completion of the reaction was confirmed by TLC on silica gel-G plates and the spots were visualized in the UV chamber or iodine chamber. IR spectra of intermediates and derivatives were recorded by using on KBr pellets on a Jasco FTIR-460 plus spectrophotometer and vibrational frequencies expressed in cm-1. 1HNMR spectra were recorded on BRUKER 400 MHz spectrometer in deuterated DMSO using tetramethylsilane (TMS) as internal standard and chemical shifts were recorded as δ (parts per million).
General Procedure:
General procedure for the synthesis of Mercaptobenzoxazole (Compound I):
2-amino phenol (10.91g, 0.1 moles) treated with carbon disulfide (7.67g, 0.1 moles) in the presence of potassium hydroxide (5.65g, 0.1 moles), 100 ml of 95% ethanol and 15 ml of water used as a solvent in a round bottom flask was refluxed on a water bath for three hours. After the completion of the reaction, the reaction mixture was cooled and filtered. After that, 1-1.5g of activated charcoal was added carefully in the filtrate and refluxed on the water bath for 10 minutes; the activated charcoal was removed by filtration. The filtrate was treated with 100ml of warm water at 60-70oC for 10 minutes. Dilute acetic acid was poured into the reaction mixture for acidification with gentle agitation to yield shiny crystals as a product, which is further kept in a refrigerator for three hours to allow the complete crystallization process. The obtained solid product was separated through the Buchner funnel and dried at 40oC overnight and recrystallized from the ethanol.
General procedure for the synthesis of substituted Mercaptobenzimidazole (DPK2B1-DPK2B4):
Mercaptobenzoxazole (5.5g, 0.1 moles) was treated with primary aromatic amine (2.7g, 0.1 moles) and was dissolved in 15 ml of glacial acetic acid. The above reaction mixture was refluxed on a water bath for 6 hours. The completion of the reaction was ascertained by TLC (Toluene: Ethyl acetate/ 1:4). After that, the reaction mixture was cooled to room temperature and filtered. The obtained solid crystals were separated through Buchner funnel and the dried product was recrystallized from the ethanol.
Table 1: Physicochemical properties of substituted mercaptobenzimidazole (DPK2B1-DPK2B4):
|
Sr.No. |
Compound code |
Molecular Formula |
Mol. wt. |
M.P.(oC) |
Percentage Yield |
Recrystallization Solvent |
|
1 |
DPK2B1 |
C14H12N2S |
240.32 |
240-245 oC |
81 |
Ethanol |
|
2 |
DPK2B2 |
C13H10N2S |
226.29 |
235-240 oC |
77 |
Ethanol |
|
3 |
DPK2B3 |
C13H9ClN2S |
260.42 |
251-256 oC |
80 |
Ethanol |
|
4 |
DPK2B4 |
C13H9N3O2S |
271.29 |
263-268 oC |
83 |
Ethanol |
General procedure for the synthesis of coupled mercaptobenzimidazole derivatives (DPK2d1-DPK2d8):
Substituted Mercaptobenzimidazole (5g, 0.02 moles) (DPK2B1-DPK2B4) treated with 2.3g (0.01 moles) dibromoalkane (1, 2- dibromoethane or 1, 3- dibromopropane) was dissolved in 60ml of ethanol. Further 5g (0.01 moles) anhydrous potassium carbonate was added as a deacidifying agent. The above reaction mixture was refluxed on a water bath for 14 hours. The completion of the reaction was ascertained by TLC (Ethyl acetate: n-hexane/4:6 or Benzene: Methanol/4:2). After that, the reaction mixture was cooled to room temperature and neutralized with 2N aqueous solution of sodium hydroxide and filtered. The obtained solid crystals were separated through the Buchner funnel and the dried product was recrystallized from the ethanol.
Table 2: Physicochemical properties of coupled mercaptobenzimidazole derivatives (DPK2d1-DPK2d8):
|
Sr.No |
Compound code |
Molecular Formula |
Mol. wt. |
M.P.(oC) |
Percentage Yield |
Recrystallization Solvent |
|
|
DPK2d1 |
C30H26N4S2 |
506.7 |
325-330 oC |
60 |
Ethanol |
|
|
DPK2d2 |
C31H28N4S2 |
520.7 |
345-350 oC |
71 |
Ethanol |
|
|
DPK2d3 |
C28H22N4S2 |
478.6 |
328-333 oC |
80 |
Ethanol |
|
|
DPK2d4 |
C32H29N5O2S2 |
579.7 |
335-340 oC |
75 |
Ethanol |
|
|
DPK2d5 |
C28H20Cl2N4S2 |
547.5 |
352-357 oC |
65 |
Ethanol |
|
|
DPK2d6 |
C32H27Cl2N5O2S2 |
648.6 |
360-365 oC |
72 |
Ethanol |
|
|
DPK2d7 |
C28H20N6O4S2 |
568.6 |
342-344 oC |
74 |
Ethanol |
|
|
DPK2d8 |
C29H22N6O4S2 |
582.7 |
355-360 oC |
68 |
Ethanol |
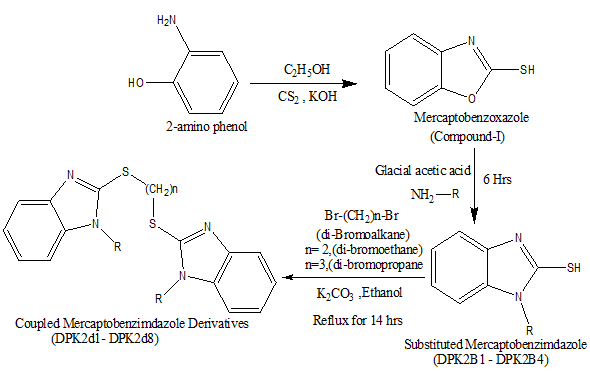
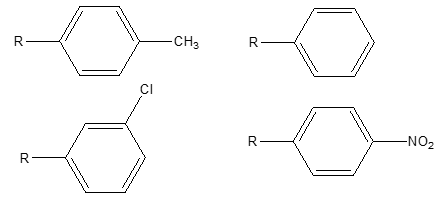
Fig.1: Synthesis of coupled mercaptobenzimidazole derivatives.
Spectral data:
Spectral data of Compound I: FTIR (KBr, cm–1): 2916 (C-H, Ar), 1651 (C=N) 1550 (C=C), 1242 (NH) 1111 (C-O-C), 779 (C-S).
Spectral data of substituted mercaptobenzimidazole:
DPK2B1: FTIR (KBr, cm–1): 3394 (NH, Benzimidazole), 3232 (C-H, Ar), 2924 (C-H, Aliphatic), 2638 (SH), 1527 (C=C), 1319 (C-N), 678 (C-S),
DPK2B2: FTIR (KBr, cm–1): 3394 (NH, Benzimidazole), 3070 (C-H, Ar), 2337 (SH), 1604 (C=C), 1319 (C-N), 694 (C-S).
DPK2B3: FTIR (KBr, cm–1): 3394 (NH, Benzimidazole), 3039 (C-H, Ar), 2507 (SH), 1573 (C=C), 1319 (C-N), 720 (C-S), 875(C-Cl).
DPK2B4: FTIR (KBr, cm–1): 3333 (NH, Benzimidazole), 3248 (C-H, Ar), 2476 (SH), 1688 (C=C), 1535 (NO2), 1388 (C-N), 717 (C-S).
Spectral data of coupled mercaptobenzimidazole derivatives:
DPK2d1: FTIR (KBr, cm–1): 3296 (NH, Benzimidazole), 3063 (C-H, Ar), 2962 (C-H, Aliphatic), 1512 (C=C), 1265 (C-N), 609 (C-S). 1H NMR (500MHz, DMSO-d6): δ 9.862 (bs, 1H, NH), 7.071-7.461 (m, 3H, Ar-H), 2.015-3.403 (m, 1H, CH).
DPK2d2: FTIR (KBr, cm–1): 3271 (NH, Benzimidazole), 3070 (C-H, Ar), 2939 (C-H, Aliphatic), 1512 (C=C), 1265 (C-N), 609 (C-S). 1H NMR (500MHz, DMSO-d6): δ 9.841 (bs, 1H, NH), 7.073-7.470 (m, 3H, Ar-H), 2.335-3.382 (m, 1H, CH).
DPK2d3: FTIR (KBr, cm–1): 3402 (NH, Benzimidazole), 3063 (C-H, Ar), 2924 (C-H, Aliphatic), 1550 (C=C), 1265 (C-N), 756 (C-S). 1H NMR (500MHz, DMSO-d6): δ 9.993 (bs, 1H, NH), 7.004-7.584 (m, 3H, Ar-H), 2.039-3.377 (m, 1H, CH).
DPK2d4: FTIR (KBr, cm–1): 3425 (NH, Benzimidazole), 3063 (C-H, Ar), 2994 (C-H, Aliphatic), 1525 (C=C), 1265 (C-N), 756 (C-S). 1H NMR (500MHz, DMSO-d6): δ 9.941 (bs, 1H, NH), 7.005-7.588 (m, 3H, Ar-H), 2.043-3.401 (m, 1H, CH).
DPK2d5: FTIR (KBr, cm–1): 3264 (NH, Benzimidazole), 3124 (C-H, Ar), 2924 (C-H, Aliphatic), 1535 (C=C), 1280 (C-N), 871 (C-Cl), 756 (C-S). 1H NMR (500MHz, DMSO-d6): δ 10.141 (bs, 1H, NH), 7.073-7.824 (m, 3H, Ar-H), 2.0362-3.381 (m, 1H, CH).
DPK2d6: FTIR (KBr, cm–1): 3305 (NH, Benzimidazole), 3070 (C-H, Ar), 2800 (C-H, Aliphatic), 1535 (C=C), 1280 (C-N), 793 (C-Cl), 702 (C-S). 1H NMR (500MHz, DMSO-d6): δ 10.138 (bs, 1H, NH), 7.075-7.826 (m, 3H, Ar-H), 2.064-3.394 (m, 1H, CH).
DPK2d7: FTIR (KBr, cm–1): 3225 (NH, Benzimidazole), 3078 (C-H, Ar), 2893 (C-H, Aliphatic), 1597 (C=C), 1311 (NO2), 1188 (C-N), 840 (C-S). 1H NMR (500MHz, DMSO-d6): δ 9.9941 (bs, 1H, NH), 6.589-7.825 (m, 3H, Ar-H), 2.123-3.362 (m, 1H, CH).
DPK2d8: FTIR (KBr, cm–1): 3483 (NH, Benzimidazole), 3039 (C-H, Ar), 2939 (C-H, Aliphatic), 1651 (C=C), 1427 (NO2), 1149 (C-N), 879 (C-S). 1H NMR (500MHz, DMSO-d6): δ 9.9841 (bs, 1H, NH), 6.586-7.954 (m, 3H, Ar-H), 2.498-3.347 (m, 1H, CH).
BIOLOGICAL STUDIES:
In Vitro Antimicrobial Evaluation:
In vitro antimicrobial activity of synthesized coupled mercaptobenzimidazole derivatives were evaluated by the tube dilution method against Escherichia coli ATCC 25922 (Gram-negative bacteria), Bacillus subtilis ATCC 6051 (Gram-positive bacteria), and Aspergillus niger ATCC 6275 (fungal strain) using Ciprofloxacin and fluconazole as standard antibacterial and antifungal drugs, respectively. All the synthesized derivatives had nine-time dilutions to be done with brain heart infusion (BHI) for minimum inhibitory concentration (MIC). Initially, 20 µL of the drugs in the initial tube was added into the 380 µL of BHI broth. For dilution, 200µL BHI broth was added into the next nine tubes separately. Further, 200µL broth from the initial tube was transferred to the first tube to make 10-1 dilution. 10-2 dilution was prepared from 10-1 diluted tube, (200 µL was transferred to the second tube). The sequential dilution was reiterated up to 10-9 dilution for each drug. 5 µL was taken from the preserved stock cultures of required organisms and was added into 2 µL of BHI broth. 200µL of the above culture suspension was added to each sequentially diluted tube. The turbidity of cultures was checked after 24 hours of incubation. The minimum inhibitory concentration was the highest dilution of the synthesized compounds (DPK2d1- DPK2d8) with no visually detectable bacterial or fungal growth.
In Vitro AntitubercularEvaluation:
In vitro antitubercular activity of synthesized coupled mercaptobenzimidazole derivatives were evaluated by the Microplate Alamar Blue Assay (MABA) against Mycobacterium tuberculosis (H37Rv strain, ATCC 27294) using Pyrazinamide, Ciprofloxacin, and Streptomycin, as standard drugs. This assay procedure was non-toxic, and used thermally stable chemical reagent. MABA method exhibited an agreeable connection with BACTEC radiometric and proportional method. During incubation, 200 µL of sterile deionized water was added to all outer perimeter wells of the sterile 96-well plate for minimizing the evaporation of the medium in the test wells. The 96-well plate in the columns received 100 µL of the Middlebrook 7H9 broth. 100 to 0.2 ug/ml of drug concentration were added to the 96-well plate. Parafilm was used to cover and sealed the plates. After that, the plates were incubated at 37oC for five days. 25 µL of a freshly prepared mixture of Almar blue reagent and 10% tween 80 (1:1) was added to the plates and were reincubated at 37oC for 24 hours. The blue colour of the well indicates that no mycobacterial growth and pink colour was counted as mycobacterial growth for synthesized compounds (DPK2d1-DPK2d8).
RESULT AND DISCUSSION:
From the literature survey, benzimidazole has been reported to develop several molecules with various potent pharmacological activities. In this research study, we have reported synthesized coupled mercaptobenzimidazole derivatives and screened for their in vitro antimicrobial and antitubercular activities. The purity and homogeneity of the coupled mercaptobenzimidazole derivatives were preliminarily checked by their physical constants. These compounds were characterized by various spectral studies such as IR, and 1HNMR for structural elucidation, and studies showed satisfactory results.
In Vitro Antimicrobial Activity:
The results of the MIC values of synthesized compounds (μg/ml) against B. subtilis, E. coli, and A. niger are summarized in Table 3. Some of the coupled Mercaptobenzimidazole derivatives were found to be highly efficient as antimicrobial agents in comparison to the standard drug ciprofloxacin (CPFX) and fluconazole (FCZ) as they represented by their low MIC values compared to standard drugs. Amongst the synthesized compounds DPK2d1 to DPK2d8 showed significant and potent activity against B. subtilis compared with the standard Ciprofloxacin. DPK2d5 showed a moderate effect against B. subtilis compared with standard Ciprofloxacin. Also, DPK2d1 to DPK2d8 showed significant and potent antifungal activity against A. niger compared with fluconazole. Whereas, DPK2d1 to DPK2d8 were found to have an average activity against E. coli compared with standard Ciprofloxacin.
Table 3: Antimicrobial activity and MIC values of synthesized compounds.
|
Sr. No. |
Compound Code |
MIC in µg/ml |
||
|
Antibacterial activity |
Antifungal activity |
|||
|
B. subtilis |
E. coli |
A.niger |
||
|
|
DPK2d1 |
0.8 |
100 |
0.8 |
|
|
DPK2d2 |
0.4 |
100 |
1.6 |
|
|
DPK2d3 |
0.8 |
100 |
1.6 |
|
|
DPK2d4 |
0.4 |
100 |
1.6 |
|
|
DPK2d5 |
3.12 |
50 |
3.12 |
|
|
DPK2d6 |
0.4 |
50 |
0.8 |
|
|
DPK2d7 |
0.8 |
50 |
1.6 |
|
|
DPK2d8 |
0.4 |
50 |
0.8 |
|
|
Ciprofloxacin |
2 |
2 |
- |
|
|
Fluconazole |
- |
- |
8 |
|
|
Control (DMF) |
- |
- |
- |
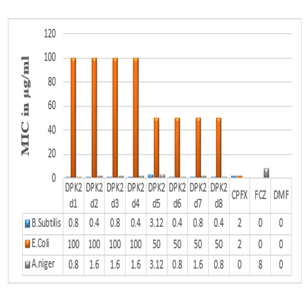
Fig. 2: Graphical representation of antimicrobial activity and MIC values of synthesized compounds.
In Vitro Antitubercular Activity:
The results of the MIC values of synthesized compounds (μg/ml) against M. tuberculosis (H37Rv) are summarized in Table 4. It is evident that among the eight compounds out of three compounds such as DPK2d1, DPK2d2, and DPK2d3 showed potent antitubercular activity with MIC of 1.6 μg/ml compared with standard antitubercular drugs such as Pyrazinamide, Ciprofloxacin, and Streptomycin.The rest of the synthesized compounds (DPK2d4 to DPK3d8) revealed moderate to good antitubercular activity.
Table 4: Antitubercular activity and MIC values of synthesized compounds.
|
Sr.No. |
Compound Code |
MIC in μg/ml |
Sr. No. |
Compound Code |
MIC in μg/ml |
|
|
DPK2d1 |
1.6 |
|
DPK2d7 |
6.25 |
|
|
DPK2d2 |
1.6 |
|
DPK2d8 |
6.25 |
|
|
DPK2d3 |
1.6 |
|
Pyrazinamide |
3.12 |
|
|
DPK2d4 |
6.25 |
|
Ciprofloxacin |
3.12 |
|
|
DPK2d5 |
6.25 |
|
Streptomycin |
6.25 |
|
|
DPK2d6 |
6.25 |
|
|
|
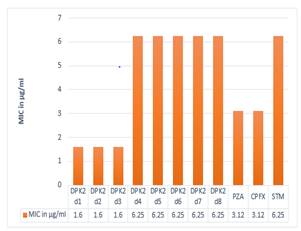
Fig. 3: Graphical representation of antitubercular activity and MIC values of synthesized compounds.
Structure-activity relationship of coupled mercaptobenzimidazole derivatives:
From the comparison of antimicrobial and antitubercular activities of synthesized coupled mercaptobenzimidazole derivatives, the following SAR may be assumed.
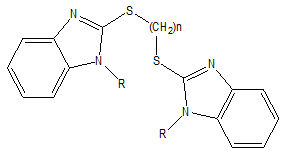
Fig. 4. SAR of coupled mercaptobenzimidazole Derivatives.
At position R:
CONCLUSION:
Coupled mercaptobenzimidazole derivatives were efficiently synthesized and screened for their in vitro antimicrobial and antitubercular activities. Amongst the synthesized compounds DPK2d1 to DPK2d8 showed significant and potent activity against B. subtilis and A. niger compared with the standard Ciprofloxacin and Fluconazole. DPK2d5 showed a moderate effect against B. subtilis compared with standard Ciprofloxacin. Also, coupled mercaptobenzimidazole derivatives such as DPK2d1, DPK2d2, and DPK2d3 showed potent antitubercular activity with MIC values of 1.6 μg/ml compared with the standard Pyrazinamide, Ciprofloxacin, and Streptomycin.
ACKNOWLEDGMENTS:
The authors would like to acknowledge Savitribai Phule Pune University, Maharashtra, India for providing a good spectral data analysis facility. We are also thankful to Dr. Kishore G. Bhat, Maratha Mandal's Central Research Laboratory Belgaum, for the antimicrobial and antitubercular screening of the synthesized compounds.
Conflict of interest:
The authors declare that they have no competing interests.
REFERENCES

