



|
Short Technological Communications Technological Development in The Production of Food Supplements
Thomas De Paoli 1*, Bruno Riccardi 2 |
|
1 Lipotech’s President, Parque Industrial La Cantabrica, Tres Arroyos 329 1706 Haedo -Buenos Aires, Republica Argentina. 2 Representative Lipotech, 56022 Castelfranco di Sotto (Pisa), Via dei lazzeri, 33, Italy. |
|
ABSTRACT |
|
In this paper, we report a general review of the nutraceutical market development of the current consumer trends and the evolution of pharmaceutical technology. In particular, we report developments and results achieved by Lipotech S.A that have allowed them to improve the effectiveness and safety of food and supplements until today. We focus on the benefits of integrating nutrition with “probiosomial” multifunctional liposomes that we have produced, which allows effective treatment of multiple nutritional deficiencies. Our technology allows the personalized administration of supplements according to specific nutritional needs obtained by Nutrigenomic's analysis using a single liposomal carrier. |
|
Key Words: Supplements, liposomal technology, probiosomial technology, Nutraceuticals. |
INTRODUCTION
Food supplements are increasingly used by a growing layer of consumers [1].
As reported by Consumer Product Reports, there is a steady increase in market demand and a parallel increase in production by companies. This phenomenon has developed especially in recent years with the widespread awareness of people that it is more important to prevent diseases, instead of treating them using drugs, which often are carriers of side effects [2]. The rule “prevention is better than cure" has been adopted by many consumers and today the benefits of prevention and risks related to the unconditional consumption of drugs are informed through the media and internet.
Today, consumers are seeking to reassess their values and priorities and focus on obtaining the most out of a good lifestyle.
In this framework, the key elements for the success of nutraceutical products, success
understood not as maximum profit, but as a maximum benefit for people, are represented by:
quality of raw materials; effectiveness and safety;
innovative environmentally friendly pharmaceutical technologies
Current production processes do not always allow to obtain optimal food supplements for quality and efficacy because of the difficulties inherent in their nature of limited solubility and assimilation or limited therapeutic margins.
This is the case of many minerals, vitamins, probiotics, and plant extracts currently used in the production of nutraceutical supplements [3]. To overcome this disadvantage, technological research has made available to biomedicine various transport systems (carriers) for drugs and active ingredients to improve absorption and tolerability.
In particular, in the biomedical field, delivery systems are intensively studied to optimize the results obtained in the diagnosis and therapy of the most common pathologies.
A recent comprehensive review of nanotechnologies and delivery systems used in the biomedical field is present in the Bozzuto-Molinari work [4].
To date, the most widely used technology is represented by liposomes for the ease of their production and versatility in their use.
Our Aim
In this paper, we report the results of many years of research devoted to improving the technology for the increasingly effective and safe production of nutraceuticals from the nutritional point of view.
In particular, research has been directed at improving the bioavailability and efficacy of those nutraceuticals, which have these properties to a lesser extent.
We outline below the essential characteristics of the productive technologies used, with an in-depth description of the liposomal technology with which we have obtained the best results.
MATERIALS AND METHODS
Among the many methods and technologies available today to improve the effectiveness and safety of food and food supplements, we report those we prefer and are widely used.
Gluconation: With this technology, the salts are gluconates and stabilized with amino acids (Certified License No. 9800574), and are characterized by excellent bioavailability, demonstrated by numerous scientific work conducted by the University of Buenos Aires, the results of which have been published in numerous scientific journals.
Microencapsulation: This technology consists of coating-covering substances and nutrients with a membrane formed by polymers of various nature, natural or synthetic.
Polymers consist of proteins, polysaccharides, polyesters, phospholipids, etc. Microencapsulation (understood as the preparation of both microcapsules and microspheres) can allow the change of color, shape, volume, solubility, reactivity, resistance, and stability of the trapped substance. The main applications of microencapsulation allow:
Liposome: Liposomes are spherical vesicles with an aqueous volume enclosed by a double phospholipid layer membrane with a structure similar to that of the cell membrane for this reason they can easily penetrate them [5]. The use of liposomes as carriers is significant for pharmacologically active substances that have a low therapeutic index (as some anticancers, antibiotics, etc.) because they make it possible to reduce the concentration of drugs and improve the bioavailability with reduction of side effects (Fig 1).
Fig. 1. Schematic comparison between Liposome and cell wall
Our first technological step: BIOFER
The first stage of our technological development was achieved by BIOFER, an iron sulfate product plus vitamin C in liposomes. Patented in 30 countries in 1994-1995, it is the only product in the world with Iron Sulphate in liposomes. In this form, Iron Sulphate is very bioavailable and free of typical side effects (Table 1).
Table 1- Biofer’s Composition (g/100ml)
|
IRON |
6.6 ± 0.3 g/100 ml / 5.5 ± 0.25 g/100g |
|
Ascorbic Acid |
1.0 ± 0,1 |
|
Sodium Ascorbate |
1.0 ± 0,1 |
|
Phospholipids |
6.0 ± 1.0 |
Manufacturing Process
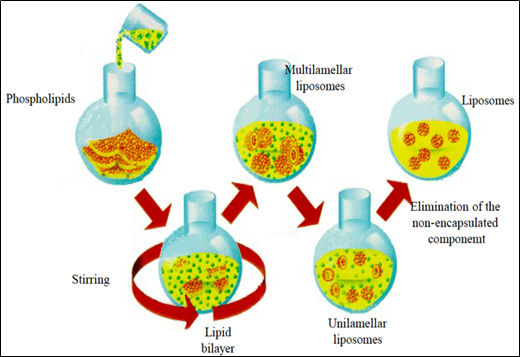
Fig. 2. Schematic representation of the production liposomes process
Although iron is one of the essential trace elements for living organisms, including man, and plays a pivotal role in numerous metabolic functions and biological synthesis, it has reduced tolerability for the important risks, which limits related to its intake, including:
low absorption, metallic taste, gastrointestinal disorders, nausea, constipation or diarrhea, and oxidative stress.
With BIOFER, all the problems related to the administration of iron have been solved.
Metabolic and biochemical studies
The metabolic pathway of iron administered as
BIOFER® was compared with one of the standard forms of iron in a metabolic study in mice. Four groups of 30 mice were given radio-labeled iron in the form of either ferrous sulphate in milk; BIOFER® in milk; ferrous ascorbate in water (molar ratio Fe/ascorbic acid = 1) or ferrous sulphate in water (under nitrogen). The last two groups were used as reference standards.
After 15 days, the animals were sacrificed and, the biodistribution of the iron was determined by using standard radiochemical techniques with the following experimental results (Fig. 3):
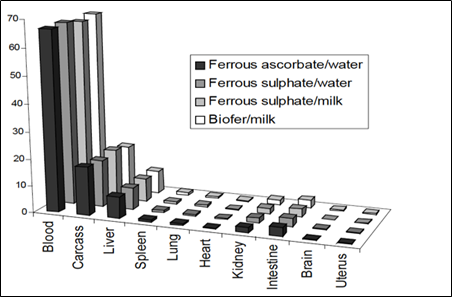
Fig. 3. Iron distribution in different organs
Iron transfer from mother to fetus
During pregnancy, a woman’s requirement for iron significantly increases as a result of her own physiological needs and the considerable quantities of iron necessary for normal fetal growth and development.
A study was carried out to investigate the distribution of BIOFER® iron during pregnancy. A group of 30 female mice was provided with milk fortified with 59Fe-labeled-BIOFER® throughout the gestation period. At birth, the percentage of iron transferred from mother to offspring was determined. The distribution of BIOFER® iron in various tissues and organs was measured (see graph below; Fig. 4. [6]).
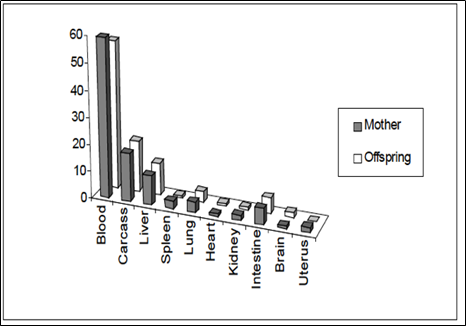
Fig. 4. Iron transfer from mother to fetus
Kinetic studies in humans
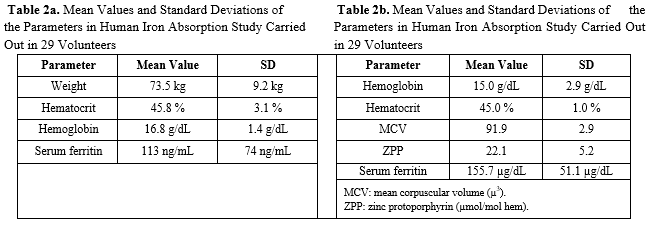
To assess the kinetics of BIOFER® iron in men, a volunteer study was undertaken.
The study was carried out on 29 physically and psychologically healthy volunteers. As shown in Table 2, the hematologic studies found that neither iron metabolism nor body iron deposits were altered. [6]
To confirm this finding, another research group carried out an absorption study on human beings to evaluate the iron bioavailability of BIOFER. The study was carried out using a modified Eakins and Brown reference
methodology [7] on a group of 15 healthy volunteers. As can be seen in Table 2, iron metabolism was not altered in any of the subjects.
The experimental results show that the geometric mean of iron absorption was 9.2%, which supports the use of BIOFER in food fortification programs [8] (Table 2).
BIOFER is currently marketed in several countries to the main multinational food companies.
Efficacy and safety of BIOFER have been documented in
numerous clinical studies reported in the bibliography [6.13].
BIOFER in supplements.
BIOFER has been used to formulate a supplement with the addition of three vitamins of B complex: Folic acid, Vitamin B6, and Vitamin B12.
The product notified to the Italian Ministry of Health with the brand Iron-Folic is marketed in Italy since 2016. In the following years, a multi-center study of Post Marketing Surveillance (PMS) was conducted in Italy.
This multi-center study brings “observational results” together from a population of over 11,000 patients treated during the years 2017-2018. PMS studies can provide valuable information about the use of the products sold in all patients who use this supplement, as well as in patients with special problems, which are not easily accessible or predictable during premarketing studies [14].
Patients were treated with 1 capsule per day of this product for three months.
All patients were asked for informed consent by physicians, who also have recorded adverse effects and any intolerances found during treatment.
The results are given in Table 3:
Table 3: The product was effective in all treated patients. The results showed that the values of Hemoglobin,
Hematocrit, Ferremia, and ferritin increased significantly (p<0.05). During treatment, adverse effects were reported in 0.20% of patients and it was not necessary under no circumstances to stop it [14].
|
|
Hematocrit (%) |
Hemoglobin (g/di) |
Ferraemia (%) |
Ferritin (ng/ml) |
Side Effetcs |
|
Initial (I) |
33 ± 1,9 |
10,1 ± 0,9 |
37,2 ± 16,1 |
12,7 ± 1,3 |
|
|
Final (F) |
38,5 ± 2,3 |
12,6 ± 0,7 |
132,6 ± 47,3 |
28,5 ± 26,4 |
0,20 % |
|
∆ (I-F) |
5,5 ± 2,9 |
2,6 ± 1,2 |
95,4 ± 48,1 |
15,8 ± 26,8 |
|
|
Tn (1) |
51,1 ± 23,5 |
50,7 ± 19,2 |
46,6 ± 26,2 |
46,7 ± 16,8 |
|
|
Pn (2) |
100% |
100% |
100% |
80% |
|
|
P% |
100% |
100% |
100% |
50% |
0,20% |
|
1) Time to reach normal values (days), (2) Number of patients who reached normal values, (3) Percentage of patients reaching normal values |
|||||
Technological Upgrading
The development of our liposomal technology had a fundamental evolutionary step in 2016 with the patent of LIFERVIT, patent N 0001423818.
With the new technology we were able to insert 5 essential nutrients in the individual liposomes: Iron Sulfate, Vitamin C, Folic acid, Vitamin B6 and Vitamin B12, and dry them in powder.
For this reason, we registered the production method under the brand name “PROBIOSOMIAL" (the meanings is multifunctional nutraceutical activity with liposomes technology).
We want to emphasize that BIOFER and LIFERVIT are the only supplements in the world that contain fundamental nutrient Iron Sulfate and other active substances in liposomes. All other products on the market contain Iron Pyrophosphate.
The association of iron sulfate with the 4 vitamins in liposome has several advantages:
Morphological investigation of BIOFER and LIFERVIT
After having produced and patented LIFERVIT, we conducted a morphological study on both products to verify whether the process of drying liposomes from the liquid to the powdered state did not change their original morphology and stability. The survey conducted at the University of Urbino confirmed the stability of liposomes to dehydrating treatment and also demonstrated the ability of liposomes to penetrate into cell cultures [25].
As demonstrated by micrographs obtained with TEM (Transmission Electron Microscopy), the spherical forms of the liposomes are clearly visible and maintain the same structure and stability from the beginning (controls) to the end of various stages of the drying process; evidence that this process does not alter the structural stability of liposomes (Fig.5) [25].
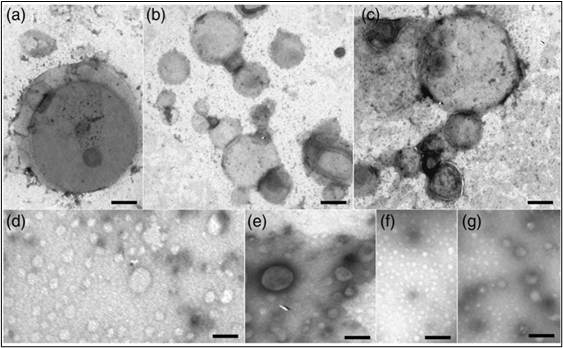
Fig. 5. Observation sample BIOFER and LIFERVIT with Transmission Electron Microscope TEM at different stages of drying
The same survey also showed the degree of absorption of liposomes by cell cultures. (Human myelomonocytic cell line U937 was grown in RPMI 1640, supplemented with 10% heat-inactivated fetal bovine serum, 2 mm glutamine, and 1% antibiotics and was maintained at 37 °C in humidified air with 5% CO2) Fig.6. [25]

Fig. 6. Up-Take of the liposome in myelocytic cell line U937 –TEM micrograph
To confirm the morphology of the liposomes, we repeated the examination of the two products at the University of Salerno with the Scanning Electron Microscopy (SEM) and the granulometric verification (Fig.7A, 7B).
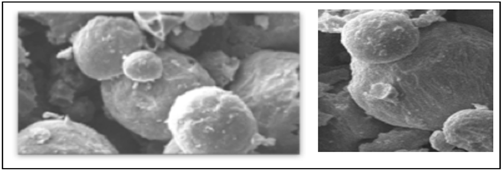
Fig. 7 A. Micrograph SEM
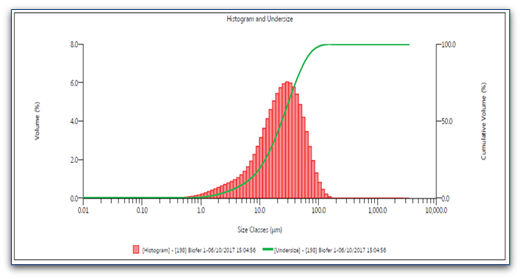
Fig. 7B. granulometric examination
RESULTS AND DISCUSSION
According to the World Health Organization: “In many parts of the world, policy-makers, health professionals and the public are wrestling with issues regarding the safety, effectiveness, quality, availability, preservation, and regulation of traditional and complementary medicine (T&CM). T&CM continues to be widely used in most countries, and its uptake is increasing rapidly in other countries. At the same time, interest in T&CM is expanding beyond products to focus on practices and practitioners. (Fig. 8) [26]
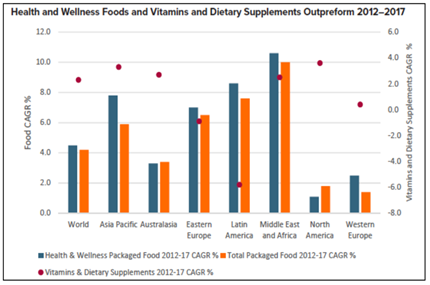
Fig. 8. Top 10 global consumer trends 2019- Euromonitor International
In the last ten years, the supplements market has grown exponentially and uncontrollably. The lack of strict regulations regarding the correct productive practice has allowed the diffusion of poor supplements both in the formulation and in the quality of the raw materials. Recently, stricter rules have been introduced for the control of supplements on the market. The monitoring is carried out by the European Food Safety Authority (EFSA), and the European Commission, which have made a decisive contribution to improving the quality of food and supplements [27].
Technology has also given important support in this direction because it allows improving the nutritional properties and safety of supplements.
Technology plays a pivotal role in consumer decision-making and the ability of manufacturers and retailers to meet the needs of today’s consumers. The constant innovation within technology and ever-faster technological processes are driving consumer’s choices.
It is important to underline how supplements can contribute to the improvement of the well-being of the population and, with the prevention of many diseases, also to reduce health costs. [16]
Through our technology, we believe to make an important contribution to promoting health and general well-being.
In particular with our Probiosomial technology we are able to formulate multifunctional liposomes containing different nutrients in their biologically active form, able to meet specific nutritional needs, and with the adoption of the principles of Nutrigenomics to promote personalized integration.
In this regard, we recall the important relationships between Nutrigenomic, Epigenetics, and nutrition.
While Nutrigenomics studies the possible interactions between the molecules introduced with food and DNA, Epigenetics shows how the introduction of food molecules can modulate the expression of DNA, the so-called phenotype, without any change in the original sequence.
Without going into the in-depth discussion of these topics that are outside the present communication, let us remember the main mechanisms that come into play in epigenetic transformations.
These are:
DNA methylation or the enzymatic addition by DNA-methyltransferase (Dnmts) family of particular methyl functional groups;
The modification of histones through acetylation, methylation, phosphorylation, etc. Acetylation, in particular, is the most common mechanism and consists of the transfer of an acetyl group to the nitrogen of the lateral chain (NH3+) histones by enzymes of the family Histone acetyltransferase (Hats).
Post Transcriptional Modifications, operated by microRNA (miRNA) that reduces the rate of transcription by the degradation of messenger RNA (mRNA).
In all these processes, the nutrients that play a fundamental role are folic acid, vitamin B6, and vitamin B12.
CONCLUSION
All the investigations and clinical work reported in our documentation demonstrate the obtainable benefits with our Probiosomiale technology with innovative multifunctional supplements, customized and formulated according to the needs to be useful for the solution of specific deficiency problems and nutritional deficits.
All these can be achieved by following the indications provided by the Nutrigenomic analysis of individual people to modulate the nutritional associations best suited to their specific needs.
Our mission is to obtain the best beneficial effects by combining our innovative technology with the best-selected diet.
We have in progress of multicenter controlled work whit our product LIFERVIT to expand and validate the experience and the obtained results with IRON-FOLIC. The results of this work will be published in the future.
Disclosure
The authors report no conflicts of interest in this work.
REFERENCES