



|
Imaging of the Male Genital Tract: A Review of the Mechanism of Sperm Quality Impairment in Infertility
Silvia W. Lestari1,2*, Andrian Japari2,3, Daniel Makes4, Gito Wasian2, Judie Hartono2, Petrus Supardi2, Andon Hestiantoro5 |
|
1 Department of Medical Biology, Faculty of Medicine Universitas Indonesia 2Andrology Specialist Program, Faculty of Medicine Universitas Airlangga/Dr. Soetomo Hospital Surabaya, Indonesia 3 Infertility Unit, Telogorejo Hospital, Semarang, Indonesia 4 Jakarta World Federation of Ultrasound in Medicine and Biology, Center of Education, Indonesia 5 Department of Obstetrics and Gynecology, Faculty of Medicine Universitas Indonesia/Dr. Ciptomangunkusumo Hospital Jakarta, Indonesia. |
ABSTRACT
Male infertility is generally diagnosed by anamnesis, physical examination, and also semen analysis. But, in some cases, imaging methods need to be used for additional help to find out the cause of infertility. A literature search from the PubMed database was performed using keywords of male genital tract imaging, sperm quality impairment, and infertility cases to find appropriate articles, then all results were collected and selected. The most common cause of male infertility is varicocele semen analysis followed by testicular atrophy, microlithiasis, undescended testis (UDT) or inflammation, which can impair spermatogenesis. Ejaculatory duct obstruction also causes blockage of sperm passage. Additional knowledge of the mechanism of sperm quality impairment, and accompanied by male genital tract imaging, is expected to help the clinician to decide more specific pathological management in andrological cases.
Key Words: imaging, male genital tract, sperm quality, andrology cases, infertility.
INTRODUCTION
Infertility is defined as an inability to conceive after regular unprotected sexual intercourse for one year. [1] In one study population revealed that 84% of women can reach clinical pregnancy in the first year of unprotected sexual intercourse regularly. [2-5] A multicentre study conducted by WHO showed that infertility can be caused by either male or female factors equally. [6] By thorough anamnesis and comprehensive physical examination, a possible cause of infertility can be determined, even if a more thorough examination is required [7].
It is estimated that men of reproductive age may experience reproductive problems, either in the testes, pre-testicular, or post-testicular. Semen analysis is the most common examination to evaluate spermatozoa quality in male infertility cases. However, sperm analysis does not always confirm the cause of infertility. The abnormalities of the semen analysis result can be due to decreased spermatozoa quantity or concentration (oligozoospermia), impaired sperm motility (astenozoospermia), and the low number of normal sperm morphology (teratozoospermia), or in combination with previously mentioned, or even absent spermatozoa (azoospermia).
Male genital tract imaging can also be used as a method of identifying causes that are not identified in a routine examination. Advances in imaging technology, especially in image quality and resolution, the use of USG (ultrasonography), the use of high-frequency sound waves for imaging, become common use in andrology clinics to evaluate male reproductive problems. [8, 9] Using Trans-Rectal UltraSonography (TRUS), Doppler ultrasonography and Magnetic Resonance Imaging (MRI) also were other options to find the cause of male infertility. This review analyzes the results of the male reproductive tract imaging commonly found in various cases of infertility, using either the mechanism or the pathophysiology of factors that impair sperm quality or abnormalities of semen analysis results. [10]
Infertility
Infertility is recognized as a condition, while married couples are not able to survive and gave birth to a live baby, and are regularly having unprotected sex for 12 months. [11] Based on the anatomy of the male reproductive tract, male infertility can be divided into three major part, namely: [12]
Just like any other diseases, the evaluation of male infertility begins with anamnesis, followed by a physical examination and, where necessary, laboratory testing. A common laboratory testing is a semen analysis, which if resulted is abnormal, indicating fertility problems. The following is a regular reference to the WHO semen analysis (Table 1). [13] Semen analysis abnormalities can be in the form of oligozoospermia, which is a decrease of sperm quantity or concentration, asthenozoospermia which is the low number of progressively motile sperm, teratozoospermia which is a small number of sperm with normal morphology, or in combination, such as oligoasthenozoospermia or even azoospermia which is characterized by the absence of spermatozoa in the seminal plasma.
Table 1: Normal Semen Reference Analysis [13]
|
No. |
Parameter Analisis Semen |
WHO 2010 |
|
1. |
Concentration |
15 million/ml |
|
2. |
Motility |
|
|
|
A. Progressive |
32 % |
|
|
B. Nonprogressive |
1 % |
|
|
C. Immotile |
22 % |
|
3. |
Morphology |
4 % |
|
4. |
Total sperm count/ ejaculate |
39 million |
Even though in evaluating male infertility we have performed anamnesis, physical examination, and laboratory tests, sometimes we still need to do more investigation to find the main cause of infertility to make a proper diagnosis, or decision about proper management, such as the genital tract imaging.
Varicocele
Abnormal dilatation of the pampiniform plexus is called varicocele. This abnormality is characterized by a presence of backflow of the vein, and in almost cases occurred on the left side. [14] Varicocele is almost absent in 11-year-old boys, but the prevalence is rising as men grew older, as high as 15% in adult men population. [15] Varicocele is differentiated into three classes: [16]
Internal spermatic vein venography has long been considered the gold standard or the diagnostic of varicocele since a long time ago, due to the smallest difference in technical variations and variability between examiners. [17, 18] Alas, this examination took longer, more aggressively as well as radiation. [18] For these reasons, venography is only indicated in limited cases nowadays. [18] In present, the American Urology/American Society for Reproductive Medicine (AUA/ASRM) is suggesting to diagnose varicocele with physical examination and then the confirmation of the diagnoses is defined by the use of ultrasonography, especially when naught can be concluded in physical examination. [19] Compared to venography, physical examinations in the diagnosis of varicocele have a sensitivity of 50-70%, whereas scrotal ultrasonography has a sensitivity of 93%. [14, 17] Due to this reason, scrotal ultrasonography becomes well accepted and widely used for the diagnosing of varicocele. [17] A study in West Africa proved that varicocele was the most common ultrasonographic abnormality in sub-fertile patients. [20]
Varicoceles are shown as an abnormal dilatation of pampiniform plexus in ultrasonography, which is characterized by the presence of backflow of the veins, which were most evident with the Valsalva maneuver. [14] This dilatation is markedly obvious by the Doppler examination (Figure 1). Based on the findings of ultrasonography, varicocele is graded into [18]
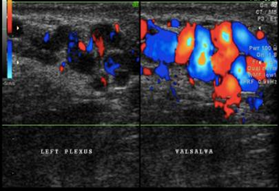
Figure 1: Varicocele (original figure with permission from radiologist)
The concept of pathophysiology mechanism of semen analysis abnormalities that disrupt the male reproductive system has been proposed by Telli et al. (2015), either by the direct mechanism that impair the process of spermatogenesis through hormonal imbalance, testicular hypoxia, and scrotal hyperthermia, or, by an indirect mechanism that raises the level of reactive oxygen species (ROS) that may lead to sperm membrane peroxidation, DNA fragmentation, and apoptosis. [21]
A study revealed that varicocele affects sperm quality, in sperm concentration and function, an elevated level of oxidative stress and DNA fragmentation. [22] A more specialized study of DNA fragmentation in varicocele also performed by Chak-Lam Cho revealed the same result with studies done previously. [23] However, research by Zini and Bowman has also shown that the opposite is true, revealing 75% of men with varicocele have a normal semen analysis. [24]
Another study showed that improvement in semen analysis after varicocelectomy was achieved, especially in sperm concentration, sperm motility, and sperm morphology. [24] These results are also justified by a meta-analysis that not only reports the same results but also shows lower levels of DNA damage and oxidative stress. [25]
Testicular atrophy
Testicular atrophy can occur after inflammation process, infection, testicular torsion, hematomas causing testicular ischemia, ischemic orchitis. [26] Ultrasonography examination can help measure testicular volume. Normal testicular volume in adult men averages 18.6 ± 4.8 ml. [27] In testicular atrophy, shrinkage in testicular volume can be evaluated. (Figure 2). [8] Other findings include a decrease in reflexivity, and testicular vascularization, although the epididymis appears normal. [28] Testicular volume below 12 ml is closely related to testicular function abnormality. [28] Laboratory examination revealed that there was a decrease in plasma testosterone levels and elevated FSH and LH levels.
Testicular atrophy also causes testosterone imbalance and estrogen levels, which results in various clinical manifestations, including gynecomastia, erectile dysfunction, infertility, and female-like pubic hair distribution patron. [29] Gynecomastia becomes the most evident proof of hormonal imbalance. [30] Low testosterone level induces diminished libido, erectile dysfunction, changes in secondary sex characteristics, and impairment of spermatogenesis process which all lead to infertility. [31] In cross-sectional histopathology of the testicular tubules, there is a marked difference between the normal testicle and the atrophic testicle whereas in normal testicle spermatogenesis is still optimal (complete set of spermatogenic cells), and in the atrophied testicle, the process of spermatogenesis is impaired (incomplete set of spermatogenic cells) (Figure 3).
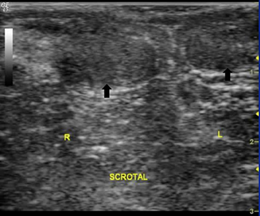
Figure 2: Atrophied testicle (original figure with permission from radiologist)
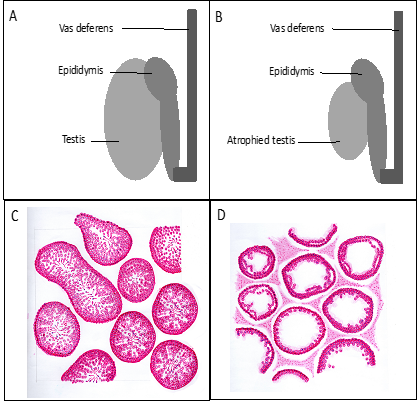
Figure 3: Volume and histopathology of the testicle. A and C. Normal testicle, B and D. Atrophied testicle (original figures provided by the first author).
microlithiasis
Testicular microlithiasis was first reported approximately 35 years ago. [32] This condition cannot be determined by physical examination, and can only be detected and diagnosed using testicular ultrasonography. Doherty et al. (1987) defined testicular microlithiasis as the presence of 5 calcification spots in one or more ultrasonographic units (Figure 4A) [32, 33] Testicular microliths are formed by several germinal cells originated from unstable spermatogenic epithelial which arose into the lumen of seminiferous tubules. Surrounding these cells, collagen layers are formed together with a calcium salt. [34] These microliths are very small and measure 1 to 3 micrometers, making it rare to find them in testicular biopsies, although they are sometimes seen occasionally. (Figure 4B) [35] Even though the diameter of the microliths is less than 1 mm, they are still too large to be able to vacate the seminiferous tubules. Nevertheless, some reports stated that testicular microliths have been observed in the rete testis, or even in the epididymis. [36]
The incidence of testicular microlithiasis in the healthy adult men population (aged 17-35 years old) is 5,6%, and the incidence is higher in the Afro-American (14,1%). [37] Microlithiasis was also associated with ethnicity and socioeconomic status. [38] (Pedersen MR et al., 2017) In boys, the incidence of microlithiasis is lower (1,1% and 4,2%). [39] Testicular microlithiasis is reported for being related to infertility, and other health-related conditions, especially in malignancy (carcinoma in situ), testicular dysgenesis, and Down’s Syndrome. [36, 40-43] In terms of infertility, one study has shown that semen analysis of infertile men is the same as the ones from fertile men with microlithiasis. In addition, the correlation between microlithiasis and primary infertility was 46% and with secondary infertility 39%, approximately 4 to 5 times higher than in the fertile men. [44]
Testicular microlithiasis may not be a serious pathological condition, but it can become a marker for testicular diseases, an indicator for testicular malignancy or benign lesion of the testicular with the potential transformation to a malignancy. [45] This might be closely related to some kind of testicular pathologies, although testicular pathologies may not be easy to prove in any patient with microlithiasis.
With the consideration that testicular microlithiasis prevalence is quite high, as high as 6%, showed that routine testicular ultrasonography would become a very important diagnostic modality. Likewise, advanced clinical management is recommended for this kind of condition, by germinal cell malignancy in the testicle, and unclassified intratubular germinal cell neoplasia. Fortunately, follow up study for 5 years revealed that none of them developed into malignancy. For this reason, a low-risk conclusion was drawn. However, routine follow up for men with microlithiasis should be performed, an advanced and thorough examination should be performed promptly if a new lesion is found.
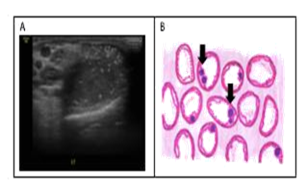
Figure 4: Microlithiasis. A. ultrasonography, B. histopathology cross-section (arrow sign shows calcification) (original figure A. With permission from radiologist and B. Made by the first author)
Orchitis and epididymo-orchitis
Infection is one of the most important factors in this field of study which mentioned in many researches. [46] Infection and inflammation of the male reproductive track can become one of the causes of male infertility. Epididymo-orchitis, either caused by local or systemic infection, or non communicable factor can affect the male reproductive function. [47] There is clear evidence that chronic inflammation in the testes can impair spermatogenesis and permanently disrupt sperm quality and concentration. Unfortunately, in most patients, the diagnosis has been made too late, due to the asymptomatic course of the disease, as well as no distinct clinical signs. Due to this reason, epidemiological data on orchitis and epididymo-orchitis is very scarce.
On the other hand, the result of testicular biopsy from infertile men showed a high prevalence of inflammation reaction. In chronic orchitis, a distinguished patron of focal and multifocal peritubular lymphocyte infiltration can be seen together with seminiferous tubules damage. These findings justify the concept that testicular inflammation induction is highly correlated with the autoimmune response which is mediated by T-cells.
The offender organism of orchitis and epididymo-orchitis most likely Neisseria gonorrhea and Chlamydia trachomatis, as well as mumps virus. [48] In Chlamydial infection, the appearance of the lesions matches the appearance of a chronic-active inflammation lesion and granulomatous which represent various stages of inflammation in chlamydial infection in other mammals. [49, 50] Still unbeknownst yet the path of this infection whether this is an ascending infection or hematogenic spreading, although the appearance of the lesions showed the inclusion of tubules epithelial cells which related to interstitial granulomatous inflammation and macrophage antigen phagocytosis followed by the damage of epithelial integrity. [51] Mild inflammation in intact seminiferous tubules – taking into account that seminiferous tubules are immune-privileged tissue – so, although there occurred complete spermatogenic cell degeneration, there is no possibility that interstitial orchitis would occur as long as the basal membrane of the tubules is still intact. Chlamydial infections in men can directly affect infertility by impairing sperm concentration, motility, morphology, and sperm DNA. [52]
Furthermore, although not a general lesion, testicular volume and Johnson’s score for spermatogenesis can be significantly reduced. In conclusion, the inflammatory reaction, although asymptomatic, can still not be taken lightly as an underlying etiology or determining factor in male infertility. Because of that, the diagnosis of epididymo-orchitis, especially asymptomatic ones need better modalities, such as testicular ultrasonography, or even testicular biopsies. [53] Testicular ultrasonography in epididymo-orchitis shows enlargement and echo-textural heterogeneity appearance, hypo and hyperechoic epididymis accompanied by testicular enlargement. (Figure 5). In Doppler, there is also hyper vascularization, both in the testicle and in the epididymis. [54]
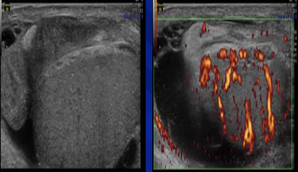
Figure 5: Epididymo-orchitis (original figures with permission from radiologist)
Ejaculatory duct obstruction (EDO)
Ejaculation is an important process in male reproductive function. The path of ejaculation involves many reproductive organs as seen (Figure 6). One of the main causes of infertility is ejaculatory duct obstruction (EDO). EDO affects 1 to 5% of infertile men and can be congenital or acquired. [55] EDO may be caused by seminal vesicle stone, Mullerian/Wolffian duct cyst, post-operative or post-inflammatory cicatrix, or even functional obstruction. In acquired cases, EDO is mainly caused by infection, inflammation, calcification, and iatrogenic. [56]
EDO can be suspected if there are found: 1) azoospermia, 2) ejaculate volume < 2 ml with pH < 7.2 and the absence of fructose, 3) normal FSH and LH serum level, 4) normal testicular volume. The diagnosis of TRUS from EDO is very useful in making the diagnosis of obstructive azoospermia and its clinical management decisions. TRUS in EDO appears as follows: 1) seminal vesicle dilatation (> 1.5 cm), 2) ejaculatory duct dilatation (> 2.3 mm), 3) cysts, calcification along the duct and 4) sperm in seminal vesicle secrete (Figure 7). Patients with congenital EDO or noninfectious EDO have a better prognosis compared to the ones with infection or complete EDO. Sperm parameters in the first group are much better than those in the next group after comprehensive management. [56, 57]
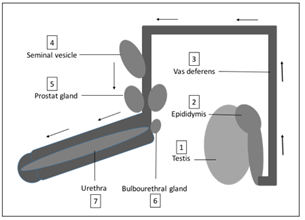
Figure 6: Ejaculatory pathway
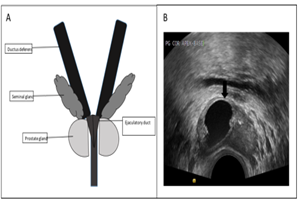
Figure 7: A. Ejaculatory duct, B. Ejaculatory duct obstruction by cyst (original figure A. Made by the first author, and B. With Radiologist's permission.
Undescended testis
Undescended testis (UDT) or commonly referred to as cryptorchidism is defined as the absence of one or both testicles in the scrotum. UDT is the most common congenital abnormality in the male genitalia. [58, 59] UDT occurs in ~ 1% of infants over three months of age. [60] Etiology of UDT is still unknown, but is considered multifactorial due to endocrine factors, environment, genetic, anatomical, and mechanical, can be implemented in the development of these disorders. [61-64] Due to the declining pathway in the fetus, as seen in the picture, UDT can occur in several locations (Figure 8A). One of the diagnostic modalities of UDT is through ultrasonography, such as inguinal UDT, as shown in the following figure (Figure 8B).
Bilateral UDT is more common in cases of infertility, compared to unilateral UDT. Research shows a decrease in sperm concentration in more than 50% of bilateral UDT men, and about 31% and 17% of unilateral UDT men. [17, 65, 66] This is due to reduced spermatogenesis in UDT. Under normal circumstances, gonadotropin and testosterone experienced an extreme increase in the third month of life. This physiological condition is the basis for the development of spermatogenic cells for optimal fertility status during growth. In the UDT, this condition is impaired so that the transformation of spermatogonia into adult dark cells (AD) (spermatogonia stem cells), is also disrupted. [67] Decreasing the number of AD cells causes the number of spermatocyte cells to decrease hence sperm concentration becomes low. [68]
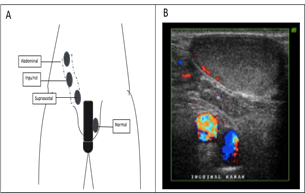
Figure 8: The undescended testicle A. Location, B. Ultrasonography (original figure A. Made by the first author and B. with radiologist's permission)
Prostatitis
Male genital tract infections such as prostatitis, epididymis, and orchitis account for 12% of the etiology of male infertility. [69] The prevalence of prostatitis is about 14%. [70] According to the US National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK), prostatitis is categorized into 4 categories: I (acute bacterial prostatitis), II (chronic bacterial prostatitis), III (chronic pelvic pain syndrome) and IV (asymptomatic inflammatory prostatitis). [71] Prostatic clinical manifestations vary, ranging from urinary disorders, pain in the genitorectal region, fever to infertility. Besides the presence of leukocytospermia, another modality for diagnosing prostatitis is ultrasonography, as shown in Figure 9A. When a biopsy of prostatitis is performed, an inflammatory reaction can be seen as shown in Figure 9B.
Complications of the prostate to infertility occur through various mechanisms such as 1) Prostate gland dysfunction, in the form of reduced fructose production, zinc to semen volume [72]; 2) Antisperm antibodies (ASA) associated with the occurrence of non-bacterial prostatitis [73, 74] ; 3) Oxidative stress, where excessive ROS can cause sperm damage [75-77] and 4) Multiple pathogens such as E. coli, C. trachomatis, mycoplasma, ureaplasma, and Enterococci are reported to be associated with impaired motility, concentration and morphology, and sperm viability. [78-80] In addition to disorders of routine sperm analysis parameters, prostatitis can also increase sperm DNA fragmentation. [80]
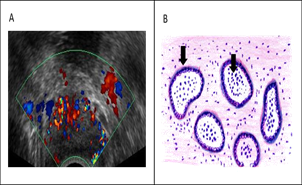
Figure 9: Prostatitis. A. ultrasonography, B. histopathology cross-section (arrow sign shows the presence of neutrophil cells in lumen and macrophages in stroma) (original Figure A with radiologist permission and B. Made by the first author)
CONCLUSION
Imaging of the male genital tract or male reproductive tract is very helpful in diagnosing andrological cases, especially in difficult cases whereas diagnosing could not be made only by anamnesis, physical examination, and routine seminal analysis. This study was performed to share information on the mechanism of sperm quality impairment that can cause infertility in andrological cases, such as varicocele, either directly caused by endocrine factors and testicular hypoxia or indirectly by formation ROS; microlithiasis, testicular atrophy, through seminiferous tubule damage which impair spermatogenesis; ejaculatory duct obstructions in which obstruct the ejaculatory path. Knowing the mechanism of sperm quality impairment accompanied by male genital tract imaging, we hope that clinicians can consider the best clinical management according to the more specific pathology.
ACKNOWLEDGMENTS
The authors would like to thank Hibah Penelitian Unggulan Perguruan Tinggi (PUPT) 2019 at Universitas Indonesia for supporting this review article. We also would like to thank Firda Asmaul Husna for helping in this review article.
REFERENCES