|
Hypoglycemic Effect of Solanumnigrum Leaf Extract on Male Diabetic Rats
Reham A. Arafat1,2, Heba A. Sindi1, Amnah M. Darwish1 |
|
1 Department of Food and Nutrition, Faculty of Human Sciences and Design, King Abdulaziz University, Jeddah, Saudi Arabia. 2 Department of Nutrition and Food Science, Faculty of Home Economics, Helwan University, Egypt. |
ABSTRACT
Diabetes (DM) is a group of complex and chronic metabolic disorders with diverse multiple etiologies. Long-term complications may affect organs such as kidneys, eyes, nerves, heart, and blood vessels. Solanum nigrum water leaves extract (SNWLE) are among the herbs used for controlling hyperglycemia in conventional medicine. This work was conducted to determine the possible effect of SNWLE on rat diabetes caused by streptozotocin (STZ). Forty adult male Wister rats were allocated to five groups (8 rats each) and studied for 8 weeks as the following scheme., group (I) normal control (cont. ( -)), group (II) diabetic control rats were given distilled water daily by gastric incubation (cont. (+) ), group (III) diabetic rats were treated orally with Glimepiride (GI) (1 mg/kg/day), groups (IV) and (V) diabetic rats were treated orally with SNWLE (250 mg/kg /day) and SNWLE (250 mg/kg /day) combined with GI (1 mg/kg/day), respectively. Blood samples and pancreas were collected on the last day of the experimental period (8 weeks) for biochemical parameters estimation as well as the histopathological examinations. The SNWLE and/or glimepiride administration to diabetic rats reduced significantly blood glucose (BG) level, kidneys function parameters, and MDA. On the other hand, insulin and CAT levels were significantly higher relative to cont. (+). Moreover, the extract showed significant amelioration in pancreatic and kidneys cells structure. These findings demonstrated that SNWLE might exert a treatment role against DM via its antioxidant mechanism.
Key Words: Diabetes, Histology, rats, antioxidant, Solanum nigrum, Streptozotocin.
INTRODUCTION
The most common metabolic disorders in the world are diabetes characterized by a decrease in insulin secretion, insulin action, or both [1, 2]. The number of diabetic patients is rapidly growing all over the world [3, 4]. According to the International Diabetes Federation (IDF), 366 million people were diabetic in 2011, with an expected increase of 552 million by 2030 [5]. The WHO stated that the prevalence of diabetes in Saudi Arabia in 2016 was 14.7% among men and 13.8% among women [6]. Diabetes has many complications such as macrovascular and microvascular diseases.
In the Kingdom of Saudi Arabia (KSA) the DM drug cost represents one of the essential economic burdens at the individual and national levels [7, 8]. Glimepiride is an oral 3rd generation sulfonylurea antidiabetic agent that contains the active ingredient that is used for the regulation of elevated blood sugar in type 2 diabetes people along with a balanced diet and workout schedule. It can also be used with other drugs for diabetes treatment [9]. Many studies are evaluating the effectiveness of alternative and natural products to overcome the suspected toxic side effects of drugs [10, 11].
Solanum nigrum (SN) was introduced in South America, Australia, and Africa and belongs to the Asian, North American, and European Solanaceae family [12]. Recent studies demonstrated that Solanum nigrum leaves have anti-diabetic, anti-hyperlipidemic properties via an increase in insulin secretion [13-14]. The leaves had been richer in polyphenols than stem and berries. The researchers noted that SN leaves contain the largest amount of luteolin, m-coumaric acid, kaempferol, gentisic acid, and apigenin [15]. Therefore, this research was conducted to evaluate the potential effect of SNWLE on diabetes.
MATERIAL AND METHODS
Material
Chemicals and Drugs
Glimepiride was obtained from Alnahdi pharmacy. Both high analytical quality chemicals and streptozotocin were purchased from Sigma, USA. The tested kits were purchased from Biosystems (Barcelona, Spain) for the determination of insulin, glucose, kidney function parameters, malondialdehyde (MDA) levels, and CAT.
Plant material
Solanum nigrum leaves have been purchased from the local market, Jeddah, Saudi Arabia.
Animals
Adult male Wister rats (n= 40) weighed 180 ± 10 g were collected from King Fahd Medical Research Centre, King Abdulaziz University.
Methods
Preparation of Solanumnigrum leaves extract.
The dried leaf powder of Solanum nigrum had been macerated with the chloroform water. The blend was stirred continuously at an interval of 3 h. After 3 days, the solvent was replaced with fresh solvent, and maceration is done 2 times, as mentioned above. The filtrate was filtered through a muslin cloth to keep away from fine powder and accompanied by Whatman No. 1 filter paper. The extract was concentrated using a rotary evaporator. Eventually, following freeze-drying, the collected SNWLE was deposited in vacuum desiccators [16].
Induction of diabetes and experimental design
Rats (n=40) were kept under control conditions in conventional animal house. They were fed a standard diet with access to water ad libitum. Rats were distributed to two main groups; first (n-=8 rats) serve as control negative group and second group DM rats (n=32) were injected intraperitoneally (i.p.) once by STZ (65 mg/kg) [17] to induce diabetes, after fasting 12 hrs. Since, the injection of STZ, rats were given a glucose solution (10 percent) for 24 hours. To fend off hypoglycemia. After 3 days, fasting blood samples were obtained from the tail vein of all rats to evaluate glucose levels. The diabetic rats were selected for the study should have blood glucose levels (>200 mg/dL) [18].
Five groups of rats were divided as follows:
Group I - Normal rats cont. (-).
Group II – DM control positive cont. (+).
Group III - DM + GI (1 mg/kg). [19].
Group IV - DM + SNWLE (250 mg/kg) [20].
Group V - DM + SNWLE (250 mg/kg) + GI (1 mg/kg).
After the experimental period 8 weeks, therapies were discontinued and rats were sacrificed under light ether anesthesia for each group. Heparinized capillary tubes used to collect blood samples, which was kept for a couple of hours, and centrifuged at 3000 rpm for 15 min. The separated serum was kept at −20˚C for subsequently followed analyzes. The pancreas was removed for histopathological studies.
Determination of serum and tissue biomarkers
All kits used were obtained from Biosystems (Barcelona, Spain).followed the steps and instructions of kits.
Histopathological examination
The pancreas from all experimental groups was removed after sacrificing the rats. Tissues were fixed in 10 percent formalin-neutral, dehydrated, coated in paraffin wax, and then stained with hematoxyl-eosin by standard procedures.
Statistical analysis
The resulting data were analyzed by SPSS version 22. Values were measured as mean ± SD and analyzed with one-way variance (ANOVA) accompanied by a t-test. The results at P≤ 0.05 were considered as statistical significance.
RESULTS
The presented data indicated that cont. (+) the group had a significant increase in serum levels of glucose by 106.36% and a significant decrease in insulin level by 73.46% compared with the cont. (-). In contrast, compared with the cont. (+) group, the data demonstrated that the treatment with Gl, SNWLE, and their mixture had a significant decrease in blood glucose by 47.31, 39.83, and 50.35% respectively, and the significant increase in insulin level by 224.39, 203.66 and 265.85% respectively (Fig.1).
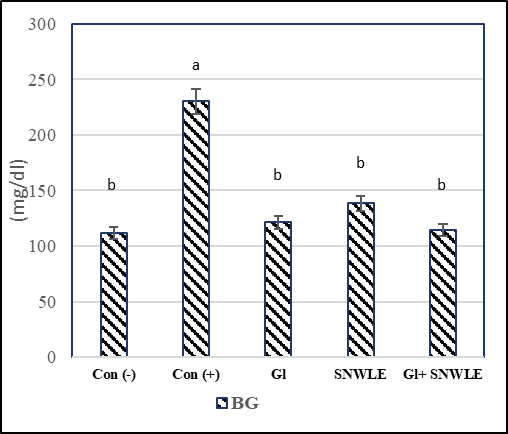
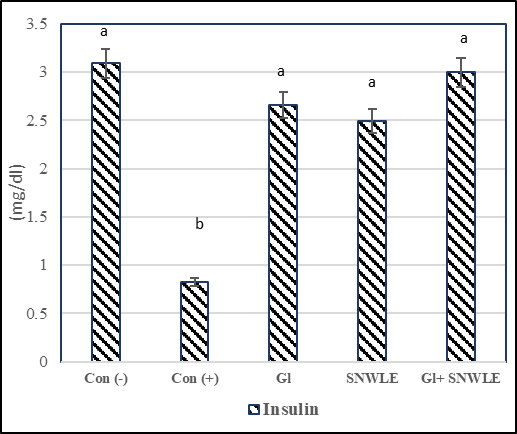
Values are presented as mean ± SD. Values with different superscript letters within a column are significantly different at P<0.05.
Figure 1: Effect of Solanum Nigrum Leaf extract (SNWLE) and/or glimepiride (Gl) on blood glucose (BG) and insulin in diabetic rats
Fig (2) showed the effect of SNWLE on uric acid (Ua), creatinine (Cr), and blood urea nitrogen (bun) in male diabetic rats. The results revealed that there were significant increases in Cr, Ua, and Bun of the cont. (+) group compared with the cont. (-) by 94.28, 101.7, and 146.49 respectively. Treatment with Gl only induced significant decreases in Cr, Ua, and Bun versus diabetic rats. Their percentages were 44.32, 45.54, and 58.87 respectively. Also, SNWLE treatment caused significant decreases by 41.89, 42.7, and 58.72 % respectively. The combination group (Gl + SNWLE) showed significant decreases in Cr, Ua, and Bun compared with the cont. (+) group by 47.73, 49.13, and 61.11 % respectively. The results confirmed that the combination group had the greatest effect rather than either each one alone.
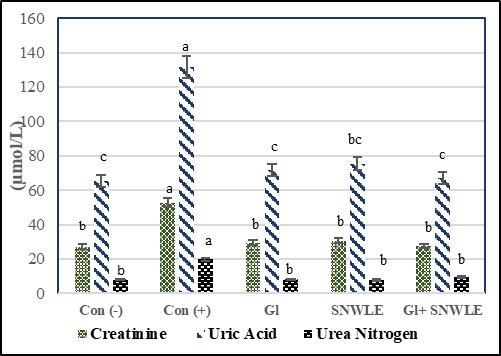
Values are presented as mean ± SD. Values with different superscript letters within a column are significantly different at P<0.05.
Figure 2: Effect of Solanum Nigrum Leaf extract (SNWLE) and/or glimepiride (Gl) on kidney function in diabetic rats.
Table (1) in the DM group, MDA (the marker of lipid peroxidation) revealed a significant elevation (p<0.005) versus the cont. (-) group. Moreover, A significant reduction in the MDA was detected with GI, SNWLE and SNWLE+ GI treated groups reverse the cont. (+) group. The most effective treatment was seen in the group treated with SNWLE+ GI. Concerning CAT results presented a significant decrease (p<0.05) in CAT in the cont. (+) group corresponding to the cont. (-); whereas treated rats with GI, SNWLE, and their mixture displayed significant elevation compared to the cont. (+).
Table 1: Effect of Solanum Nigrum Leaf extract (SNWLE) and/or glimepiride (Gl) on malondialdehyde (MDA) and catalase (CAT) in diabetic rats
|
Groups |
MDA µmol/g protein |
CAT nmol/min/mg protein |
|
Cont (-) |
73.46 ± 3.27 ᶜ |
188 ± 1.36 ᵃ |
|
Cont(+) |
118.36 ± 4.51ᵃ |
146 ± 2.30 ᵇ |
|
GI |
85.55 ± 2.76 ᵇᶜ |
178 ± 1.82 ᵃ |
|
SNWLE (250 mg/kg) |
88.41 ± 1.67 ᵇ |
175 ± 1.66 ᵃ |
|
SNWLE (250 mg/kg) +GI |
74.08 ± 3.72 ᶜ |
183 ± 1.75 ᵃ |
Values are presented as mean ± SD.
Values with different superscript letters within a column are significantly different at P<0.05.
Histopathological results
The negative control group's pancreas revealed normal pancreatic cells Fig. (A). Meanwhile, the positive control group's pancreas revealed necrosis of Langerhan's islet cells Fig. (B). pancreas cells of GI treated diabetic rats showed slight congestion of glomerular tuft Fig (C). While, TheSNWLE group treated with two hundred and fifty mg/kg of diabetic rats, the pancreas revealed slight hypertrophy of Langerhans islets Fig. (D). Pancreas sections of rats given the mixture did not show any change in histopathology Fig. (E).
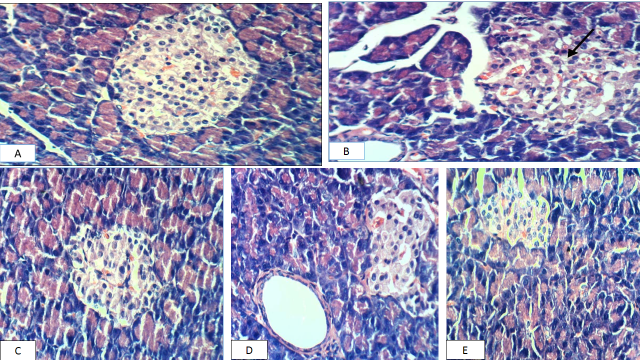
Figure 3: Photomicrography showing H&E-stained sections of the pancreas in different groups. The control negative pancreas, displaying normal pancreatic cells (A). In diabetic rats, parts of the pancreas showed necrosis of Langerhan's islet cells (B), pancreas cells GI treated diabetic rats showed slight congestion of glomerular tuft (C). The SNWLE group treated with two hundred and fifty mg/kg of diabetic rats, pancreas revealed slight hypertrophy of Langerhans islets (D), Pancreas sections of rats given the mixture did not show any change in histopathology (E). (x400)
DISCUSSION
Diabetes is recognized as one of the leading causes of morbidity and mortality in the world, while about 8% of the world's population has been diagnosed with DM in 2017, it is still predicted to grow to 9.9% by 2045. [21]. As evidenced by the fact that prescription drugs such as insulin-like substances are medicinally recognized as the most effective therapeutic agents, researchers have sought to find insulin-like substances based on plants to treat diabetes [22]. The clinical trials confirmed the efficacy of several medicinal plants and herbal preparations in improving the natural homeostasis of glucose [23]. Several kinds of research demonstrated that hyperglycemia induces oxidative stress and increases the production of ROS [24, 25]. Several studies revealed the benefits of medicinal plants and herbs such as solanum nigrum which shows antidiabetic and hypolipidemic effects [26-28].
Recent results from the study showed that the DM group showed a substantial increase (p < 0.05) in serum glucose, followed by a significant reduction in serum insulin relative to the cont. (-). These findings agree with Emordi et al. [29] Who reported that STZ-diabetes hurt glucose concentration and insulin level in rats with a dosage of 60mg / kg. These findings could be explained by insulin secretion inhibited by STZ which could induce insulin-diabetes Mellitus due to its unique chemical properties, which is called alkylating potency [30], the β- cell destruction and insulin resistance [31].
Nagrashi et al ., [32] recorded that the administration of 50 mg/kg b.wt., streptozotocin to rats causing diabetes by raising serum glucose levels and decreasing insulin levels this may be due to the injection of streptozotocin, which induced diabetes to animal models by exerting cytotoxic effects on pancreatic β-cells, likely to generate lipid peroxide and excess reactive oxygen species (ROS), interfere with GLUT-2 glucose carriers, and cause alkylation or peroxynitrite harm to DNA [33].
Treatment with SNWLE and/or glimepiride (Gl) for diabetic rats demonstrated a remarkable improvement effect on glycemic regulation. Such findings are in the same line with Maharana et al. [20] Who suggested that Solanum nigrum extract had hypoglycaemic and antihyperglycaemic activity because of its possible effect on the glucose and lipid metabolism of pancreatic and extra-pancreatic sites, as demonstrated by the extract's insulinotropic and antioxidant properties. Ogunsuyi et al., [34] demonstrated that SNWLE had a hypoglycemic activity that may result from two mechanisms first: potentiation of glucose-induced insulin release, second: increasing peripheral uptake of glucose.
Data in the recent study indicated that there were significant increases (p <0.05) in serum creatinine and uric acid levels in diabetic untreated rats corresponding to the cont. (-) group which strongly indicated the impairment of kidney function in DM rats. These results were in agreement with Khanraet al., [35], Qiao et al., [36] and Zayed et al., [37]. The rise in kidney functions may be attributed to metabolic disruptions during diabetes attributed to a rise in xanthine oxidase activities, increased TC, and TG levels [38].
Although the oral administration of SNWLE and/or glimepiride (Gl) to diabetic rats in the current study caused significant decreases (p<0.05) in the levels of uric acid, urea, and creatinine corresponding to cont. (-) group. The data obtained were in the same line with Teklehaimanot et al. [39] who indicated that the reduction of serum uric acid, urea, and Cr levels in SNWLE treated groups could be due to kidney glomeruli regeneration, which improved the kidney filtration function. The use of antioxidant and/or free radical scavenging will control the nephroprotective functions. This defensive and regenerating capacity could be due to the high SNWLE content of polyphenols, alkaloids, and saponins [40].
Regarding antioxidant activity, the results showed a significant increase in MDA levels accompanied by a significant decrease in serum CAT enzyme activity in the cont. (+) group corresponding to the cont. (-) group. The obtained data agreements with Nasri et al., [41] and Afify et al., [42]. Also, Morakinyo et al., [43] confirmed that DM rats administered STZ at a dose level of 45 mg/ kg adversely affected the function of MDA, SOD, GSH, GPX, and CAT enzymes. The changes of antioxidant parameters may be due to an increase in oxygen species (ROS) involved in DM production and progression [44].
In the current study, the results reported that there was a significant increase in CAT enzyme activity while MDA demonstrated a significant decrease in the diabetic group treated with SNWLE and/or glimepiride (Gl) reverse the Con. (+). These study findings were conforming with the results of Ayatullah et al., [45]. and Syed et al., [14]. Moreover, Maharana et al., [26] stated that SNWLE might have antioxidant compounds that can reverse the damage caused by diabetes.
In conclusion, the SNWLE has significant protective effects against diabetes and hyperlipidemia via its antioxidant property.
REFERENCES

