



|
Green Synthesis, Characterization and Antibacterial Activity of Zinc Nanop articles Using Stem Extract of Lawsonia inermis Linn
Madhavan Ramyajuliet1, Gnanaraj Gnana Priyanka Beaulah2, Pious Soris Tresina3, Asirvatham Doss3, Veerabahu Ramasamy Mohan3* |
|
1 Research Scholar (19212232262038), PG & Research Department of Botany, V.O.Chidambaram College, Thoothukudi, Tamil Nadu, India, Affiliated to Manonamaniam Sundaranar University, Tirunelveli. 2 Research Scholar (18112232262009), PG & Research Department of Botany, V.O.Chidambaram College, Thoothukudi, Tamil Nadu, India, Affiliated to Manonamaniam Sundaranar University, Tirunelveli. 3 Ethnopharmacology Unit, V.O.Chidambaram College, Thoothukudi, Tamil Nadu, India. |
ABSTRACT
In the production of nanoparticles, the bio-reduction properties of plant extracts are acknowledged worldwide to reduce the harmful effects of physical and chemical methods of synthesis of nanoscale metal particles. Zinc nanoparticles (ZnNPS) have been widely employed for various pharmacological applications. The present study recommends a green approach for the synthesis of zinc nanoparticles employing aqueous extract of stem of Lawsonia inermis. The biosynthesized ZnNPs were conformed visually by the appearance of white color formation in the mixture. The prepared ZnNPs were characterized by UV-via absorption, FTIR, SEM, XRD and AFM analysis. The UV-visible spectra showed that the absorption peak existed at 332nm. FTIR analysis revealed that phenolics, carboxylic acid, aromatic and amines present in the stem extract are responsible for the reduction and stabilization of the ZnNPs. SEM analysis demonstrated that the synthesized ZnNPs were spongy like shape. The XRD results indicated that the synthesized product showed hexagonal wurtzite phase and the average particle size is 17.80nm. The AFM image of ZnNPs exhibited a mixture of rod and spongy type of structure. The ZnNPS exhibited the antimicrobial activity against gram (+) bacteria, Bacillus thuringiensis, and Streptococcus faecalis: L. inermis may be used for the green synthesis of ultrafine nanoparticles of zinc for their antibacterial activities.
Key Words: Zinc nanoparticles, UV-Visible, FTIR, X-ray Diffraction, AFM.
INTRODUCTION
The growth of green processes for the blend of nanoparticles has been evolving into an essential branch of nanotechnology as green nanotechnology compacts with secure and eco-friendly ways for nanomaterials fabrication and this is considered as an alternative to conventional physical and chemical methods [1, 2]. For the past two decades, the research on synthesized nanomaterials and their distinct features is a new field of nanotechnology because of the wide applications in fields like chemistry, physics, biology, and medicine [3, 4]. Even- though physical and chemical methods are more popular for nanoparticle synthesis, the use of toxic compounds, bounds their applications [5]. Definitely, over the past numerous years, plants, algae, fungi, bacteria, and viruses have been utilized for the production of metallic nanoparticles [6, 7]. Sometimes the amalgamation of nanoparticles using plants or parts of plants can establish advantages over other biological processes by eliminating the complicated processes of upholding microbial culture [8].
Using environmentally suitable plant extract and eco-friendly reducing and copping agents green nanoparticle synthesis has been reached. The use of plants for synthesis of nanoparticles is rapid, low cost, eco-friendly and a single-step method for the biosynthesis process [9]. Plant and plant materials have become a potential source for the synthesis of metallic nanoparticles recently. Many researchers have reported on the synthesis of metallic nanoparticles including silver, gold, titanium dioxide, tungsten oxide and copper oxide [10-14] using different plant materials. But reports on an amalgamation of zinc nanoparticles using plant materials are limited [15].
Lawsonia inermis Linn. (Family: Lythraceae) is a branched glabrous shrub or tiny tree (2-6 m in height), grown for its leaves while stem bark, roots, flowers, and seeds have also been employed in conservative medicine. This plant is a wide-reaching known superficial agent used to stain hair, skin, and nails [16]. The plant has reported the following properties: Analgesic, anti-inflammatory, antibacterial, wound healing, antimicrobial, hypoglycemic, immunostimulant, hepatoprotective, antifungal, antiviral, antiparasitic, antitrypanosomal, antioxidant, tuberculostatic, antidermatophytic, antifertility and anticancer properties.
The leaves of Lawsonia inermis are used to take care of poliomyelitis, measles among the Yoruba ethnic group of South Western Nigeria [17]. The seeds of henna have been accounted to possess deodorant action which is used in most cases of gynecological disorders like menorrhagia, vaginal discharge and leucorrhoea [18]. Henna is extensively used in the cosmetic industry as a dyeing agent as well in India [19]. Reports demonstrate that methanolic root extracts of Lawsonia are used in Nigeria as an antimalarial drug [20] in addition to abortifacient purposes [21]. There is ginger oil that is mixed with the powdered roasted seed to build a paste that is used for the managing ringworm. To heal wounds and clean wounds aseptically, the decoction of the leaves is used. [22]. L.inermis is also made use of by some individuals as ‘blood tonic’, thus implying its multifaceted application [20]. Hence the present study aims to develop a novel approach for the green synthesis of zinc nanoparticles using herbal plant Lawsonia inermis stem extract.
MATERIALS AND METHODS
Collection of Plant Material
Lawsonia inermis Linn. was collected from Pullavali, Pazhayakayal. The collected samples were cut into tiny fragments and shade dried in anticipation of the fracture is uniform and smooth. The dried material was granulated or powdered by using a blender, and sieved to get uniform particles by using sieve No. 60. The finishing uniform powder was utilized for the extraction of energetic constituents of the plant material. The plant samples were found with the help of local flora and approved by the Botanical Survey of India, Southern Circle, Coimbatore, Tamil Nadu, India.
Preparation of Extracts for Phytochemical Screening
Hot Maceration Method Using Soxhlet Apparatus
Newly collected L. inermis stem was dried in shade, and then coarsely powdered in a blender. The coarse powder (100 g) was extracted successively with petroleum ether, benzene, ethyl acetate, methanol, ethanol and water, every 250 ml in a Soxhlet apparatus for 24 hrs. Through Whatman No.41 filter paper all the extracts were filtered. The entire extracts (petroleum ether, benzene, ethyl acetate, methanol, ethanol and aqueous) were focused on qualitative tests for the identification of different phytochemical constituents according to the standard procedures [23-25].
Green Synthesis of Nanoparticles
Preparation of Stem Extract (Reducing Agent)
To prepare the stem extract, 20 gm of L. inermis stem was taken. Using double distilled water, the stem was thoroughly washed and then cut into fine pieces. Fine pieces were boiled in 100 ml double distilled water for 20 minutes at 60oC in a glass beaker. Using Whatman No. 1, the extract was filtered after boiling.
Preparation of Precursors
Precursors for zinc nanoparticles (ZnCl2) were purchased from Hi-media chemicals, India and prepared freshly. Precursor for preparing zinc nanoparticle was 0.02 M of zinc chloride using double distilled water.
Synthesis of Zinc Nanoparticles
10 ml aqueous solution of stem extract was slowly added to 10 ml of 0.02 M zinc chloride solution under constant stirring. Afterward, the mixture was treated with 1 M sodium hydroxide drop by drop. Soon after this, the mixture was changed into pale white. This was then kept in a magnetic stirrer for two hours. The white precipitate was obtained and dried in a hot air oven overnight at 60°C. Complete conversion of Zn (OH)2 into Zn nanoparticles took place during drying.
CHARACTERIZATION OF THE SYNTHESIZED ZINC NANOPARTICLE
UV – Vis Spectroscopy
Ultraviolet-visible spectroscopy (UV-Vis) submits to absorption spectroscopy in the UV-visible spectral area. The zinc nanoparticles were characterized in a Shimadzu V 650 UV- Vis spectrophotometer. The scanning range for the samples was 300-700 nm. The double distilled water is made use as a blank reference.
Fourier Transform Infra-Red Spectroscopy (FTIR)
The nanoparticles were illustrated utilizing a Fourier Transform Infrared Spectrophotometer (FTIR Thermoscientific iS5). Two milligrams of the sample was mixed with 100 mg Potassium bromide (KBr). Compressed to prepare a salt disc roughly 3 mm in diameter and the disc were at once kept in the sample holder. FTIR spectra were recorded in the absorption range between 400 and 4000 cm-1.
Scanning Electron Microscope (SEM) Analysis
SEM is a kind of electron microscope that images a sample by scanning it with an elevated energy beam of electrons in a raster scan models. On a carbon-coated copper grid, this film of the sample was prepared by just dropping a very small amount of the sample on the grid. To remove the extra solution, a blotting paper was used and then the films on the SEM grid were permitted to dry by keeping it below a mercury lamp for 5 min.
X-Ray Diffraction (XRD) Analysis
The particle size and nature of the zinc nanoparticles were determined using XRD. This was done using the Shimadzu XRD – 6000/6100 model with 30 kV, 30 mA with Cukα radians at 2θ angle. X-ray powder diffraction is a fast analytical technique. This is chiefly used for phase identification of a crystalline material and can supply information on unit cell dimensions. The analyzed material is thinly ground, and the normal bulk composition is established. The particle or grain size of the zinc nanoparticles was determined using Debye Sherrer’s equation.
D= 0.94 λ/ Bcos θ
AFM Analysis
The surface topology of the synthesized zinc nanoparticles were studied by 1µm x 1 µm Atomic Force Microscopy (AFM Nanosurf 2) analysis, 0.01 g synthesized nanoparticles were mixed with 20 ml of acetone and sonicated for 5-10 minutes using ultrasonicator. The solution was poured on a clean glass slide and was allowed to dry until all the acetone gets evaporated. To study this glass slide, the Atomic Force Microscopy is used in a non-contact mode. To process the captured image, XEI software was used.
Antibacterial Activity
The antibacterial activity of synthesized nanoparticles was determined using a disc diffusion method [26]. The test bacteria Bacillus thuringiensis, Streptococcus faecalis, Salmonella paratyphi, and Escherichia coli was obtained from the Research Laboratory, Department of Microbiology, Bharathidasan University, Tiruchirapalli, Tamil Nadu. The overnight incubated bacterial culture was spread over the freshly prepared nutrient agar plates. The 6 mm sterile disc (Hi media) was kept at the center and different concentrations of synthesized nanoparticles (40 µg/mL, 80 µg/mL and 100 µg/mL) were poured on disc and placed on the plate. The tetracycline disc (reference or positive control), ZnCl2 solution without extracts and plant aqueous extract were also kept and then incubated at 37° C for 24h and after incubation the zone of inhibition was measured.
RESULTS AND DISCUSSION
Preliminary Phytochemical Analysis
The results of preliminary phytochemical screening of various solvent extracts of L. inermis stem were presented in Table 1. The methanol ethanol and aqueous extracts of L. inermis stem showed the presence of alkaloid, catechin, flavonoid, phenol, saponin, steroid, tannin, glycoside and xanthoprotein. Different phytochemicals were detected from L. inermis stem extracts which makes the plant useful for the treatment of different ailments. It also has the potential of providing useful drugs for human use.
Synthesis of ZnNPs by Green Synthesis Process
The reaction was visible as a color change from light yellow to white within 24 hrs of the addition of reaction mixture Figure 1 showed the difference in the colour of extract and synthesized ZnNPs.

Figure 1: Synthesis of Zinc Nanoparticles from L.inermis Stem
a) Plant Extract b) Zinc Chloride c) Zinc Nanoparticles
Characterization of Synthesized ZnNPs
UV-Vis Spectroscopy Analysis
The nanoparticles were primarily characterized by UV-Vis spectrophotometer, which was proved to be a very useful technique for the analysis of nanoparticles. The absorption spectrum was recorded for the synthesis of ZnNPs in the range of 200-900nm by a UV-visible spectrophotometer. The white color formation is the characteristic of ZnNPs formation and also depends on their Surface Plasmon Resonance (SPR). The spectrum showed the absorbance peak at 332nm corresponding to the characteristic of ZnNPs (Fig 2). Vimala et al. [27] reported on the absorbance spectra of ZnO nanoparticles which were recorded between 330nm and 370nm. The above findings were supported by present studies.
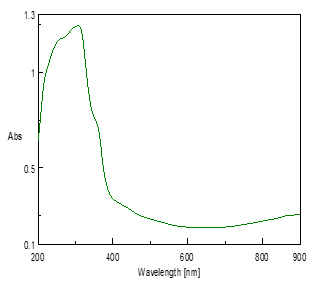
Figure 2: UV-Visible Spectrum of Zinc Nanoparticles of L. inermis Stem
Fourier Transform Infra-red Spectroscopy (FTIR)
The FTIR spectrum was utilized to recognize the functional groups of the lively components based on the peak value of the region of infrared radiation. The peak values of different compounds present with their frequencies and functional groups were shown in Table 2. The FTIR spectrum of the stem powder of L. inermis, (Figure 3) which clearly shows the peak at 3407 cm-1 corresponds to the O-H stretching of a hydroxyl group, peak at 2923 cm-1 represent O-H stretching of carboxylic acid, peak at 2852 cm-1 assigned as C-H stretching of alkanes, peak at 1625 cm-1 represent >N-H bond of a secondary amine, peak at 1443 and 1381 cm-1 represent C-O stretching of esters and ethers, peak at 1224 and 1153 cm-1 assigned as C-N stretching of aliphatic amines, peak at 778 cm-1 represents C-H ‘oop’ of aromatics, peak at 617 cm-1 assigned as C-Br stretching of alkyl halides. Figure 4 shows the FTIR spectrum of the biosynthesized zinc nanoparticles, peak at 3397 cm-1 corresponds to the O-H stretching of alcoholic or phenolics, peak at 2923 cm-1 represents O-H stretching of carboxylic acid, peak at 1597 cm-1 assigned as C-C stretching of aromatics (in-ring), peak at 1493 cm-1 represents C-C stretching of aromatics, peak at 1493 cm-1 represent C-C stretching of aromatics, peak at 1383 cm-1 corresponds to the C-H rock of alkanes, peak at 1023 cm-1 represent C-N stretching of aliphatic amines, peak at 880 cm-1 represent C-N‘oop’ of aromatics.
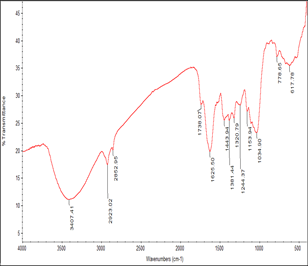
Figure 3: FT-IR Spectrum of Stem Powder of L. inermis
Figure 4 shows the FTIR spectrum of the biosynthesized zinc nanoparticles, peak at 3397 cm-1 corresponds to the O-H stretching of alcoholic or phenolics, peak at 2923 cm-1 represents O-H stretching of carboxylic acid, peak at 1597 cm-1 assigned as C-C stretching of aromatics (in-ring), peak at 1493 cm-1 represents C-C stretching of aromatics, peak at 1493 cm-1 represent C-C stretching of aromatics, peak at 1383 cm-1 corresponds to the C-H rock of alkanes, peak at 1023 cm-1 represent C-N stretching of aliphatic amines, peak at 880 cm-1 represent C-N ‘oop’ of aromatics.
FTIR measurement was performed to identify the biomolecules/organic molecules responsible for capping, reducing and stabilizing the ZnNPs present in the stem extract of L. inermis. The obtained results suggest the presence of various functional groups in the stem as well as in synthesized ZnNPs.
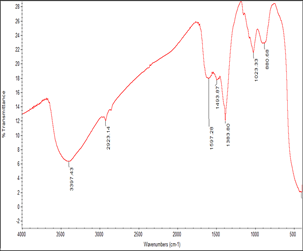
Figure 4: FT-IR Spectrum of Zinc Nanoparticles of L. inermis Stem
The stem extract has a variety of metabolites such as alcoholic or phenolics, carboxylic acid, alkane, secondary amine, aromatics, esters, ethers, aliphatic amines and alkyl halides which are responsible for the antioxidant or reducing property that aids in the immediate reduction of zinc ions into nano-structured ZnNPs. From the FTIR spectra of L. inermis stem extract and sample, there was an observed change in wavenumber of the functional groups that was due to the reduction and stabilization of metal group Zn [28].
Scanning Electron Microscopy Analysis of ZnNPs (SEM)
The morphology and growth features of prepared ZnNPs were examined using scanning electron microscopy. Figure 5 showed the SEM image of the ZnNPs synthesized using L. inermis stem extract. The picture substantiates the spongy like the structure of the ZnNPs. From the SEM images, the crystallite size of ZnNPs synthesized using L. inermis stem extract was formed to be in the nanometre range.

Figure 5: SEM Image of Zinc Nanoparticles of L. inermis Stem
X-Ray Diffraction (XRD) Analysis of ZnNPs
XRD pattern of ZnNPs showed the peak corresponding to Bragg’s diffraction signals from the crystal plants (111), (111), (111), (200), (210), (211), (211), (211), (211) and (211). The intensity data were assembled starting from 20° to 80° over an array of 2q. A definite line broadening of the XRD peaks indicates that the prepared materials consist of particles in the nanoscale range (Figure 6). The diffraction peaks located at 31.97°, 34.66°, 47.76°, 56.72°, 63.06°, 66.51°, 68.07°, 72.72° and 77.01° have been indexed as hexagonal wurtzite phase of ZnNPs. Furthermore, it proves that the synthesized nano-powder is pure as it does not have any characteristic XRD peaks other then ZnNPs. From the XRD peaks, the estimation of average particle size was calculated using Scherrer’s equation at 17.80nm.
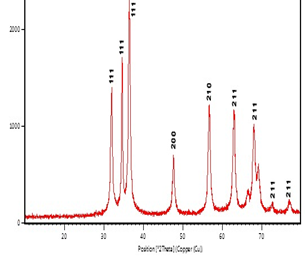
Figure 6: XRD Pattern of Synthesized Zinc Nanoparticles
Atomic Force Microscopy (AFM) analysis of ZnNPs
The surface topology of the synthesized ZnNPs was studied by 1µm×1µm atomic force microscopy (AFM) analysis. AFM was used as the primary method to monitor ZnNPs dissolution and agglomeration pattern. The topography of micrographs cleanly indicates that the formulated ZnNPs posses a mixture of rod and spongy shape as shown in Figure 7. The synthesized nanoparticles were stable in air and water and did not connect to any other associated compounds. Hence it exists highly dispersed nanoparticles.
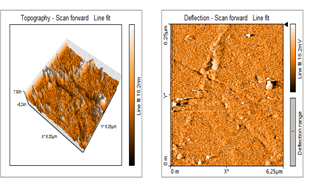
Figure 7: AFM Structure of Zinc Nanoparticles of L. inermis Stem
Antibacterial Activity
Zinc nanoparticles (ZnNPs) were synthesized by using L. inermis stem extracts for the antibacterial activity against two gram (+)bacteria such as Bacillus thuringiensis and Streptococcus faecalis and two gram (-) bacteria like Salmonella paratyphi and Escherichia coli. ZnNPs have been formed highly toxic against gram (+) bacteria such as B. thuringiensis and Streptococcus faecalis (Table 3).
What accounts for the enhanced antibacterial activity of nanoparticles is the increase in their surface area which is available for interaction. This enhances the bactericidal effect than the large-sized particles thereby imparting cytotoxicity to the microorganisms [29]. Researchers have not completely understood the mechanism that the nanoparticles used to penetrate the bacteria, but studies have shown that a change took place in its membrane morphology of the bacteria that were treated with zinc nanoparticles and this membrane morphology produced a noticeable increase in its permeability which affects proper transport through the plasma membrane [30, 31] leaving the bacterial cells incompetent of properly regulating transport through the plasma membrane, effecting into cell death. In the study, it was discovered that the zinc nanoparticles that have penetrated inside the bacteria caused damage by interacting with phosphorous and sulfur that contain compounds such as DNA [32, 33].
The result of numerous investigations have shown the possible mechanisms that involve the interaction of nanomaterials with the biological macromolecules. It is believed that microorganisms carry a negative charge while metal oxides carry a positive charge. This creates an ‘electromagnetic’ attraction between the microbe and the treated surface. ZnNPs synthesized from L. inermis stem extract proved to be a more potent antibacterial activity.
In conclusion, environmentally benign and low-cost synthesis of zinc nanoparticles can be achieved using the stem extract of L. inermis. The synthesized zinc nanoparticles exhibited good antibacterial activity. Therefore, nanoparticles of zinc in amalgamation with commercially available antibiotics could be utilized as an antibacterial agent after additional trials on experimental animals.
ACKNOWLEDGMENTS
The authors acknowledge Kalasalingam University, Krishnankoil for rendering their support to carry out the SEM analysis and the Department of Physics, Manonmaniam Sundaranar University, Tirunelveli to carry out the XRD analysis.
REFERENCES