




Medicinal herbs are plants that have medicinal properties for various diseases and also maintain human and animal health, as a result of the concerns about the side effects of conventional medicine, the use of natural products as an alternative to conventional treatment in the healing and treatment of several diseases has been increased in the last decades. This study aimed to evaluate the effect of different levels of Ginger and Mentha on the immune changes in rats injected with carbon tetrachloride (CCl4). The experiment was performed in the animal house. The rats were fed on a basal diet before starting the experiment for 1 week, then divided into 2 main groups, the first group (n= 4) was fed on the basal diet only as a control negative normal rats for 28 days. The rats of the second main group (n= 32) were injected with CCl4. The second main group was divided into eight sub-groups, including three groups fed with different concentrations (5%, 10%, and 15%) of Mentha and three groups fed with different concentrations (5%, 10%, and 15%) of Ginger and one group mixed of all plants and one control positive group infected with the disease and did not feed on the experimental diet. The results demonstrated that group 8 (rats fed on a diet containing 15% Mentha) showed the lowest level in lymphocytes among all treatment groups and recorded the best results compared to the normal group. Moreover, the result showed that there was no significant difference among groups 3, 4, and control positive groups. Groups 5, 7, and 9 showed similar (P>0.05) mean values of hematocrit. Finally, group 6 (rats fed on a diet contain 5% Mentha) and group 8 (rats fed on a diet containing 15% Mentha) showed the lowest levels of hematocrit among all treatments and recorded the best results compared to the normal group.
INTRODUCTION
Immunity is the body's ability to recognize and eliminate foreign materials. Hence, the immune system is the body's natural defense system against foreign substances that have penetrated the skin or mucous membranes. Accordingly, the immune system's response to the hormonal and metabolic changes that accompany stress, however, is to suppress its disease-fighting activity [1]. If malnutrition accompanies stress, the immune system will force to work without adequate nutrient support, further impairing its activity. Impaired immunity raises the risk of disease, disease impairs nutrition, and poor nutrition impairs immunity [2]. The immune system is composed of a very interactive and complex network of cells and their products. The system has 2 unique characteristics: "memory" and exquisite specificity, that are, the capability of the immune system in mounting a much more effective and vigorous response in the second time a specific stimulus is received, and a subset that the immune cells recognize and respond to each of the myriads of external stimulation that a person may encounter in a lifetime respectively The immune system regulates itself so-called helper suppressor cells. It is also in close communication with other systems in the body (e.g., the neuroendocrine system) and is regulated by those systems as well [3]. The immune function of the human body undergoes adverse changes with age. The T-cells that have a key role in cellular immunity, exhibit the largest age-related differences in function and distribution, with the involution of the thymus as the main obvious reason. The immune response to acute exercise has not extensively been studied in the elderly. The response of natural killers (NK) to a single exercise challenge is normal in the elderly, but immediately after exercise, the elderly individuals manifest less suppression of Phyto hemagglutinin (PHA)-induced lymphocyte proliferation than younger individuals. In contrast, strenuous exercise appears to induce a more sustained post-exercise suppression of cellular immunity in the elderly than in their young peers. Some cross-sectional comparisons of immune status between young sedentary control individuals and physically fit elderly cases showed that habitual physical activity increase NKs activity, checking certain aspects of the age-related T-cell function declines including decreased production of certain cytokine and decreased mutagenesis in response to plant lectins. However, clinical implications should be clarified in the future [4].
Chronic liver damage is a prevalent disease that is characterized by a progressive evolution from steatosis to chronic hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma [5]. As oxidative stress plays a role in liver disease pathogenesis and progression, the use of antioxidants has been suggested as drug coadjuvants and therapeutic agents to improve liver damage [6-8].
Ginger (Zingiber officinale) is a flowering plant whose root, ginger, or rhizome is extensively used as folk medicine and spices is a herbaceous perennial that grows annual pseudostems (false stems made from the rolled leafs bases) about 1m tall bearing narrow leaf blades. The inflorescences bear flowers directly arise from the rhizome on separate shoots, having pale yellow petals with purple edges [9]. Ginger is in the family Zingiberaceae that also includes galangal, cardamom (Elettaria cardamomum), and turmeric (Curcuma longa) It originated in Island Southeast Asia and was probably first domesticated by the Austronesian people. During the Austronesian expansion (c. 5,000BP), it was transported across the Indo-Pacific and reach as far as Hawaii. This plant is a spice that was exported from Asia, arrived in Europe, and used by ancient Romans and Greeks. The distantly related dicots in the genus Asarum due to their similar taste are called wild gingerproduction was 2.8million tons in 2018, with India leadin with 32% of the world total [10].
Mentha is a genus of the Lamiaceae family with aromatic and medicinal value. It includes 25-30 species that widely grow in temperate regions worldwide, especially in the Near East (Ethiopia, Syria), Northern parts of Iran, Asia Minor, Europe, North Africa, and North America, but today, it is cultivated worldwide. Mentha piperita is a prototypical member of the mint family, normally quadrangular, and 50–90cm high [11].
Aım of study
This work aimed to show the probable benefit of different levels of Ginger and Mentha on the immune changes in rats injected with CCl4.
MATERIALS AND METHODS
Materials
preparation of ginger and Mentha: ginger and Mentha were cleaned thoroughly by washing, cut into small slices, and dried in a drying oven at 50°C for 3 days, then crushed and milled as fine powder.
Experimental animals: 36 male albino rats, Sprague Dawley strain, weighing 150±10g were used in the study.
Used chemicals: CCl4 was obtained from El-Gomhoryia Company for Chemical Industries, Cairo, Egypt as a 10% liquid solution dispensed in bottles each containing for liver poisoning [12]. it is mixed with paraffin oil obtained from the pharmacy for dilution during the induction.
Methods
Biological experiment
Basal diet composition of rats
The basal diet in the test contained starch (69.5%), com oil (10%), casein (10%), vitamin mixture (1%), salt mixture (4%), choline chloride (0.2%), methionine (0.3%), and cellulose (5%) [13] (Table 1).
Table 1. Composition of basal diet
|
Ingredients |
Amounts |
|
Protein (casein) |
10%* |
|
Corn oil |
10% |
|
Mineral mixture |
4% |
|
Vitamin mixture |
1 % |
|
Cellulose |
5% |
|
Choline chloride |
0.2 % |
|
Methionine |
0.3 % |
|
Corn starch |
Up to 100% |
Source: Reeves et al., (1993).
Data in Table 2 the basal diet in the test contained CaCO3 (600 mg), K2 HPO4 (645 mg), Ca HPO4. 2H2O (150 mg), MgSO4.2H2O (204 mg), Nacl (334 mg), Fe (C6H5O7) 26H2O (55 mg), Kl (1.6 mg), MnSO4.4H2O (10 mg), Zncl2 (0.5 mg) and Cu SO4. 5H2O (0.06 mg) [14] (Table 2).
Table 2. The composition of salt mixture (g/100 g)
|
Compounds |
Amount |
|
CaCO3 |
600 mg |
|
K2 HPO4 |
645 mg |
|
Ca HPO4. 2H2O |
150 mg |
|
MgSO4.2H2O |
204 mg |
|
Nacl |
334 mg |
|
Fe (C6H5O7) 26H2O |
55 mg |
|
Kl |
1.6 mg |
|
MnSO4.4H2O |
10 mg |
|
Zncl2 |
0.5 mg |
|
Cu SO4. 5H2O |
0.06 mg |
Source: [14]
Table 3 showed the basal diet in the test contained Vitamin E (10 Iu), Vitamin K (0.50 Iu), Vitamin A (200 Iu), Thiamin (0.50 mg), Pyridoxine (1.00 mg), Niacin (4.00 mg) Calcium panthothenic acid (0.40 mg), Vitamin D (100 Iu), Choline chloride (200 mg), Folic acid (0.02 mg) , Inositol(24 mg), Para-amino – benzoic acid(0.02 mg), Vitamin B12(2.00 µg) and Biotin (0.02 mg) [13] (Table 3).
Table 3. The composition of vitamin mixture
|
Vitamin |
Amount |
|
Vitamin E |
10 Iu |
|
Vitamin K |
0.50 Iu |
|
Vitamin A |
200 Iu |
|
Thiamin |
0.50 mg |
|
Pyridoxine |
1.00 mg |
|
Niacin |
4.00 mg |
|
Calcium panthothenic acid |
0.40 mg |
|
Vitamin D |
100 Iu |
|
Choline chloride |
200 mg |
|
Folic acid |
0.02 mg |
|
Inositol |
24 mg |
|
Para-amino – benzoic acid |
0.02 mg |
|
Vitamin B12 |
2.00 µg |
|
Biotin |
0.02 mg |
Source: [13]
Induction of liver intoxication in rats
28 rats were injected with subcutaneous CCl4 in paraffin oil 50% V/V (2ml/kg BW) 2times a week for 2weeks to induce chronic liver damage as described by Jayasekhar et al., (1997). Then, blood samples were collected by the retro-orbital method to estimate liver function and ensure liver injury
Experimental design and animal groups
In the study, thirty-six Sprague Dawley male albino rats (150±l0g) were used. Rats were housed in wire cages under normal laboratory conditions and fed on a basal diet for one week as an adaptation period. To avoid contamination or loss of food, the diet was given in non-scattering feeding cups, water was given using glass tubes projecting through the wire cage from an inverted bottle supported to one side of the animals were divided into 9groups each of 4 rats. The groups of rats were as follows:
Biological evaluation
During the experimental period, the consumed feed and body weight were recorded weekly. The body weight gain (BWG%), food efficiency ratio (F.E.R), and organs weight were determined according to [15].
Blood sampling
Blood samples were collected by the retro-orbital method using microcapillary glass tubes, into a dry clean centrifuge tube and left to clot in a water bath 37 ºC for 30min. The blood was centrifuged for 10 minutes at 3000rpm to separate the serum for glucose determination and the rest was aspirated and transferred into clean quit fit plastic tubes and stored at (-20ºC) until analysis. The organs (spleen, heart, kidney, and liver) were removed and washed by saline solution, weighted, and kept in (10%) formalin solution [16].
Biological evaluation
Food intake (consumption), BWG (%), and feed efficiency ratio (FER) ) were calculated according to [15] using the following equations.
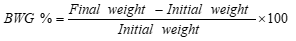 |
(1) |
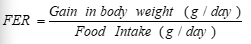 |
(2) |
|
|
(3) |
Biochemical analysis
Complete blood count (CBC) test
The test is included WBC, HB, RBC, WBC, and platelet count. The results of CBC are generated by highly automated electronic and pneumatic analyzers based on aperture-impedance andor laser beam cell sizing and counting according to Jacobs et al. (2001) [17]:
Statistical analysis
Data were analyzed statistically using SPSS software effects of different treatments were analyzed by One-way ANOVA test using Duncan’s multiple range test and p<0.05 was [18].
RESULTS AND DISCUSSION
This work aimed to show the probable benefit of different levels of ginger and Mentha on the immune changes in rats injected with CCl4.
biological results: Effect of different levels of ginger and Mentha on the immune changes on rats injected with(CCl4)
Table 4 shows the effect of different levels of ginger and Mentha on Hemoglobin, red blood cells, platelets, lymphocytes, and Haematocrit on rats injected with CCl4.
Table 4. Effect of different levels of Ginger and Mentha on the immune changes on rats injected with (CCl4)
|
Parameters Groups |
Hb (mg/dl) |
RBC (mg/dl) |
Plat (mg/dl) |
Lypho (mg/dl) |
Hemato (mg/dl) |
|
Group 1 (Control -ve) |
11.4±0.67a |
4.08+0.11A |
395.3±1.75a |
38.3+0.58c |
37.1+0.3a |
|
Group 2 (Control +ve) |
9.4±0.32c |
3.5±0.39b |
200.7+10.4e |
44+3.6a |
30.3+1.03° |
|
Group 3 (5% Ginger) |
10.9±0.4b |
3.76+0.1 5b |
210.9+0.09° |
37.7+0.58° |
32.7+0.35° |
|
Group 4 (10% Ginger) |
9.9±0.27c |
3.55±0.27b |
207.8±2.3d |
40.7+0.538 |
30.3+0.52° |
|
Group 5 (15% Ginger) |
10.7±0.02b |
3.72±0.33b |
210.7+3.7° |
37.3+0.73° |
33.8+0.11B |
|
Group 6(5%Mentha) |
11.4±0.67a |
4.19+0.76a |
220.7+11.7C |
36.7+1.05c |
36.7+0.57a |
|
Group7 (10% Mentha) |
10.9±0.21b |
3.77±0.02b |
211.1+0.34° |
37.3+0.35c |
34.8+1.32b |
|
Group8 (15%Mentha) |
11.9±0.32a |
4.4+0.05a |
227.3+1.76b |
31.6+1.35° |
36.7+0.71a |
|
Group 9 (15% mixture of all plant) |
10.3±0.04b |
3.65+0.048 |
207.3+3.45° |
37.4+0.56c |
34.7+0.768 |
Hemoglobin (Hb)
Table 4 and Figure 1 shows the effect of feeding CCl4-intoxicated rats with tested materials on Hemoglobin. It is clear that the Hb value was 9.4±0.32 mg/dl in rats injected with CCl4 (control positive group)In normal rats (control negative group), the mean value of Hb was 11.4±0.67 mg/dl. These results revealed that there was a significant decrease in Hb of rats poisoned by CCl4 as compared to normal rats. The mean value of Hb in rats given then fed on all diets in groups 3, 4, 5, 6, 7, 8, and 9 was 10.9±0.4, 9.9±0.27, 10.7±0.02, 11.4+0.67, 10.9±0.21, 11.9+0.32, and 10.3+0.04 mg/dl, respectively showing a significant difference as compared to the positive group except G4. There is no significant difference between G4 and the control positive group as well as G6, G8, and normal group. Groups 3, 5, 7, and 9 showed similar (P>0.05) mean values of Hb. Meanwhile, rats fed on a basal diet containing 15% Mentha (G8) showed the highest increase in Hb level and recorded the best results as compared to all treatments. This result is in agreement with Shahram, et al., (2012) they who explained the effect of different various levels of peppermint (Mentha piperita) plant powder, on the immune system of broilers under heat stress conditions, They 192 one-day-old chickens (Ross, 308) were randomly allocated to 4 dietary treatments with 4 replicates of 12 chicks each, using a completely randomized design. results indicated reported that supplementation of peppermint powder in the diet did not improve the weight of bursa of Fabricius spleen and bursa of Fabricius spleen in weight of broiler chicken, but had an antioxidative potential effect to improve immune response and oxidative stability. and immune response.
|
|
|
Figure 1. Effect of different levels of Ginger and Mentha on the Hemoglobin (Hb) on rats injected with (CCl4) |
Red blood cells (RBC)
As shown in Table 4 and Figure 2, the red blood cells significantly decreased in the control positive group as compared to the normal rats (3.5±0.39 and 4.08+0.11 mg/dl, respectively). There was no significant difference among groups 3, 4, 5, 7, 9, and control positive group, which were 3.76±0.15, 3.55±0.27, 3.72±0.33, 3.77±0.02, 3.65±0.04, and 3.5±0.39, respectively. Also, there was no significant difference between G6, G8, and control negative groups, which were 4.19±0.76, 4.4±0.05, and 4.08+0.11 mg/dl, respectively. Meanwhile, group 8 (rats fed on a basal diet with 15% Mentha) showed that there is no significant difference in red blood cells as compared to normal rats and recorded the best treatment.
|
|
|
Figure 2. Effect of different levels of Ginger and Mentha on Red blood cells (RBC) on rats injected with (CCl4) |
Platelets
Data presented in Table 4 and Figure 3 revealed that the injection of CC14 led to a significant (P<0.05) decrease in the platelet level in hepatotoxic rats. The mean ± SD of platelets in the hepatotoxic control positive group was 200.7±10.4 mg/dl as compared to 395.3+1.75 mg/dl in the control negative group [19-23]. The mean value of platelets in rats given CC14 then fed on all diets of groups 3, 4, 5, 6, 7, 8, and 9 which were 210.9+0.09, 207.8±2.3, 210.7±3.7, 220.7±11.7, 211.1+0.34, 227.3+1.76 and 207.3+3.45 mg/dl, respectively showing significantly higher than control positive group. There was no significant difference among groups 3, 4, 5, 7, and 9. Finally, Rats fed on a basal diet contained 15% Mentha (G8) showed the highest increase in the platelet level and recorded the best results as compared to all treatments.
|
|
|
Figure 3. Effect of different levels of Ginger and Mentha on Platelets on rats injected with (CCl4) |
Lymphocytes
As shown in Table 4 and Figure 4, that rats injected with CC14 (control positive group) had a higher (P<0.05) value of lymphocytes compared to the normal rats (control negative group), which were 44±3.6 and 38.3±0.58 mg/dl, respectively. The mean value of lymphocytes in rats given CC14 then fed on all diets of groups 3, 4, 5, 6, 7, 8, and 9 which were 37.7±0.58, 40.7±0.53, 37.3±0.73, 36.7±1.05, 37.3±0.35, 31.611.35, and 37.4±0.56 mg/dl, respectively showing significant decreases as compared to the positive group. There was no significant difference among groups 3, 5, 6, 7, 9, and normal rats. Meanwhile, group 8 (rats fed on a diet contained 15% Mentha) showed the lowest level of lymphocytes among all treatments and recorded the best results as compared to the normal group.
|
|
|
Figure 4. Effect of different levels of Ginger and Mentha on Lymphocytes on rats injected with (CCl4) |
Hematocrit
Data in the Table 4 and Figure 5 Explained that rats injected with CC14 in (control +ve group) had a hematocrit value of 30.3+1.03 mg/dl. In normal rats (control -ve group), the mean value of serum hematocrit levels was 37.1+0.3 mg/dl. These findings denote that there was a significant decrease in hematocrit of rats poisoned by CC14 as compared to the normal rats. The mean values of level hematocrit in rats given CC14 then fed on all diets of groups 3, 4, 5, 6, 7, 8, and 9, which were 32.7+0.35, 30.3+0.52, 33.8+0.11, 36.7+0.57, 34.8+1.32, 36.7+0.71, and 34.7+0.76 mg/dl, respectively were significantly higher than the positive control group. There was no significant difference among groups 3, 4, and the control positive group. Groups 5, 7, and 9 showed similar (P>0.05) mean values of hematocrit. Finally, group 6 (rats fed on diet contained 5% Mentha) and group 8 (rats fed on diet contained 15% Mentha) showed the lowest levels of hematocrit among all treatment and recorded the best results compared to the normal group. The mechanism by which the mint induces its hepatoprotective activity is not certain. However, it is possible that (3- sitosterol) is a component of ginger, which is partly responsible for the protective activity against CC14 hepatotoxicity [24]. Besides, the recorded content of vitamin C in the ginger (35-38 mg per 100g) may also play a role in hepatoprotection showing that the metabolism of hepatic microsomal drugs decreases in ascorbic acid deficiency increases when large amounts of vitamin supplements are given [25].
|
|
|
Figure 5. Effect of different levels of Ginger and Mentha on Hematocrit on rats injected with (CCl4) |
CONCLUSION
We found that both ginger and Mentha had a strong effect in improving the immune status of mice injected with carbon tetrachloride, and the improvement rate increased in the group containing a mixture of ginger and Mentha, due to the presence of flavonoids in both plants, which is a factor that contributes to the protective ability of the liver by inhibiting cytochrome P-450 aromatase A.
Recommendatıons
Acknowledgments: None
Conflict of interest: None
Financial support: None
Ethics statement: None