|
Certain Extracellular Productions in Bacillus subtilis Cultures Supplemented with Banana Waste as Substrate
Ikram-ul Haq 1*, Imranullah Jatoi 1, Nazia Parveen Gill 2, Sikandar Ali Sangrasi 3, Hadiqa Komal 1, Noman Ali 1 |
|
1 Institute of Biotechnology and Genetic Engineering (IBGE), University of Sindh, Jamshoro-76080, Pakistan. 2 Deparment of Statistics, University of Sindh, Jamshoro-76080, Pakistan. 3 Institute of Physiotherapy and Rehabilitation Sciences, Liaquat University of Medical and Health Sciences (LUMHS), Jamshoro-76090, Pakistan. |
ABSTRACT
The Bacillus subtilis (k1) grew in submerged fermentation cultures supplemented with various organs of banana agricultural wastes as the cheapest substrate. Due to the differential richness of cellulose, hemicellulose, and lignin of banana organs, it could be a valuable factor for B. subtilis growth and the production of valuable secondary products. The B. subtilis was inoculated on LB0 (TY-medium), LB1 (⅛ TY-medium), LB2 (LB1 + leaf blade), LB3 (LB1 + leaf midrib), LB4 (LB1 + leaf sheet), LB5 (LB1 + cobe leaflets), LB6 (LB1 + fruit peel) and LB7 (LB1 + sucker) cultures supplemented with 15 % and 30 % extracts of each banana organ. After 12th hrs of culture incubation (37°C, 250 rpm). All Bacillus cultures have shown variant growth rates except LB1. Maximum reducing sugars were observed on LB2a (0.442±0.041 mg ml-1), LB4a (0.444±0.012 mg ml-1) and LB6 (0.444±0.007 mg ml-1), while total proteins on LB3a and LB5a. Meanwhile, free prolines and glycine betaine observed maximum in the LB2 and LB3a cultures respectively. The highest enzyme activities were also noted as pectinase in LB6a, amylases in LB7a, cellulases LB2a, lipase LB4, and proteases LB7, while pectinases and lipases remained maximum over others among the cultures at the harvest time (p≤0.05). These findings have shown that each organ of banana waste has different fueling potential, which could be a useful substrate for the biosynthesis of hydrolytic industrial enzymes with bacterial fermentation.
Key Words: Bacillus subtilis, submerged fermentation, banana waste, hydrolytic enzymes, free proline.
INTRODUCTION
The enzymes are the most important potential biocatalysts among the living organisms for their specific metabolic reactions [1, 2]. The production of environmental eco-friend enzymes and their purifications are the central aims of today’s modern biotechnology industry [3, 4]. The fermentation has remained a very important approach for such bio-productions of cell biomass and secondary metabolites either with wild or genetically engineered microbial strains [5]. Both solid-state fermentation (SSF) and submerged fermentation (SMF) have been used for the synthesis of many products including enzymes, antibiotics, peptides and growth factors [6]. With SMF more than 75% of industrial enzymes have produced under optimal bacterial growth conditions [7].
The Bacillus species have been considered as a SAFE organism for human and it is being a natural horse for tremendous extracellular heterologous protein producer. It exports the proteins directly into an extracellular medium which increases the easiness in downstream processing of proteins [8–10]. Around 60% of commercial enzymes are produced with this group of bacteria, which is an ultimately leading important industrial organism over other bacterial species. Both of their easy cultivation and safe economic extra-cellular productions are bringing this class of highly favorable bacteria for the food industry [11, 12]. A large variety of extracellular hydrolyzing enzymes like amylases, proteases, and xylanase have been widely ranging of usage in the food, feed additive, chemicals and pharmaceutical industries [13].
Despite running the expensive and toxic chemical reactions, its replacement with echo friend enzymes is gaining significant importance in the industries due to its reduced cost in the manufacturing of products [14]. Many enzymes are performing in a great way like xylanases are among the most important industrial enzymes. They have been used for the production of chemicals, treatment of animal feeds, used as a food additive in industries of baking, bio-bleaching and as an ingredient in textile industries for washing and fabric care [15]. Similarly, the proteases are used in various industries of waste management, diagnostics, pharmaceuticals, silver recovery, detergent, food and leather [16]. Also, the lipases are a well-known group of versatile enzymes equipped with a variety of natural functions of hydrolyzing carboxylic ester bonds. Due to this characteristic, they are the potential agent in pharmaceuticals, detergents, leather, cosmetics, food, textile, and paper industries [17].
Many of the extracellular hydrolytic biocatalysts are useful in the management of environmental wastes including agricultural vegetative wastes also. The microbial pectinases and cellulases are involved in the breaking down of pectin (cementing agent of the plant cell wall) and cellulose (major complex carbon stuff of plant cell wall) respectively [14, 18–20]. Similarly, tannases are hydrolyzing the gallic acid into tannins (widely produced in various microorganisms and plants) which useful in beverage, food, cosmetic and pharmaceuticals [21].
Among the agricultural wastes, the banana (Musa sp.) is also contributing it’s all vegetative parts including the peels of the fruit also [22, 23]. It has been estimated that a large quantity of agricultural waste produced by a banana plant, which is discarded either into nearby rivers or by burning them which causes environmental problems [24]. This agricultural waste can be utilized for the production of various industrial products including the enzymes, as it is rich with a good quantity of hemicellulose, cellulose and lignin previously reported as 25.23%, 28.92%, and 10.56% respectively [25, 26]. Even different organs of banana wastes are rich with various carbon compounds [27]. The targets of modern biotechnology include recycling and use agricultural wastes as a nutrient source in the microbial cell growth cultures.
By keeping in view, the above reports, Bacillus subtilis is selected as a fermentation organism, which is capable to utilize banana waste materials into valuable synthetic industrial products including enzymes. The productions of cost-effective industrially important enzymes by Bacillus subtilis through sub-merged fermentation on banana agricultural wastes are observed.
MATERIALS AND METHODS
Microorganism and preparation of inoculum
The Bacillus subtilis (k1) was taken from frozen glycerol stock at -40°C. It was activated in 2 ml LB₀ [TY-medium: 10 g l-1 tryptone, 5 g l-1 yeast extract, 5 g l-1 NaCl and pH 7.0] medium by incubation at 37°C with constant 250 rpm shaking incubator for overnight [28]. Its 0.2 ml was inoculated in 250 ml fresh LB₀ medium and incubated in the same shaking incubator for 1 hour. This culture was used as a master culture to develop other cultures supplemented with banana agriculture waste.
Preparation of banana waste fermentation cultures
Fresh vegetative banana plants were harvested by the farmers and thrown away as agricultural waste. They were searched out in the vicinity of the institute. Plants were selected and its various parts (i.e. leave sheet, leaf midrib, fruit peel, floral leaflets, leaf blade, and sucker) were excised. These collected banana plant organs washed in tap-H2O, moistened and chopped into the small pieces than grinded with a blender in an equal volume of autoclaved dH2O (w/v) properly. The mixture was squeezed with a muslin cloth and finally filtered by using Whatman filter paper (product number Z274852). The extracts were stored in plastic bottles at 4°C for the next use.
Fermentation cultures
The LB₀ medium was diluted to ⅛ strength with dH₂O (v/v) and labeled as LB₁ medium. It was used as a nutrient deficit control medium in the experiment. The extracts of different organs were mixed in LB₁ medium @ 15% and 30% including two controls one with LB₀ medium and another LB₁ without substrate (Table 1). All the media with and without substrate were sterilized at 121°C for 20 minutes and allowed to cool at room temperature.
Inoculation of fermentation organism
The sterilized and cold medium as prepared according to the composition given in table 1 were used. These media inoculated with a fresh master culture of Bacillus subtilis (k1) with the final 0.02 initial OD600 of all cultures. The cultures were incubated at 37˚C with 250 rpm shaking for 12 hours.
Harvesting the cell cultures of Bacillus subtilis
The fermentation cultures were harvested after 12 hrs of incubation under specific Bacillus culture conditions. The OD600 of each culture was taken to measure the cell growth rate than centrifuged at 5000 rpm for 10 minutes. Its supernatant was separated and used as the crude enzyme sample. The pellet was re-suspended in 1 ml 35% H2O2. Both typed samples were stored at 4°C for the next use.
Biochemical analysis
Various bio-products were analyzed in the collected cultures. Like total sugars [29], reducing sugars [30] and total proteins [31] were determined following the methods reported by different research groups. The total protein contents were determined by mixing equal volume of sample with 5% Na2CO3 (v/v) than 25% volume of above mixture folin-ciocalteu’s reagent was added at room temperature. The absorbance was read at 760 nm against blank [32]. The ascorbic acid and flavonoids were determined [33, 34].
The free amino acids like as glycine betaine and proline were also quantified. For glycine betaine 2 ml sample mixed with 0.75 ml 1M potassium iodide the incubated at 65°C for 15 minutes. Its OD365 was taken again against blank [35]. For proline, 1 ml sample mixed with 1ml CH3COOH, 1 ml acidic ninhydrin and 2 ml toluene. After incubation at boiling water bathed for 1 hour, the mixture was cooled down at room temperature than OD540 was taken [36, 37]. Similarly, antioxidant activity was determined by mixing 1 ml sample with 2ml reaction reagent (0.588ml of H2SO4, 0.049g (NH4)2MoO4 and 0.036g Na3PO4). The reaction tubes were capped with aluminum foil and incubated at 95°C in a water bath for 90 minutes. After cooling at room temperature, OD695 was taken against blank [38]. The total fructose contents [39] and phosphates were also determined [40].
Measurements of enzyme activities
The same supernatant sample cultures were also subjected to the analysis of enzyme activities like as for xylanase activity, 1 ml sample mixed with an equal volume of freshly prepared enzyme substrate (1% xylose in citrate buffer pH 5.3) than 2 ml DNS was added. The OD540 was taken against blank [41]. For invertase, 1 ml sample mixed 1 ml enzyme substrate (1 g sucrose dissolved in 0.02 M Acetate buffer pH 5.0). After 15 min of incubation at 35°C, 2 ml DNS was added, and the reaction mixture was kept in a boiling water bath for 5 minutes. The absorbance was read at OD540 against blank [42]. The protease [43], lipase [44], pectinase [30], cellulase [30, 45] and amylase [46–48] activities were also determined.
Statistical analysis of data
The collected data on culture growth parameters, various biochemical analyses and enzyme activities were analyzed by using ANOVA (analysis of variance) and DMR (Duncan’s multiple ranges) to determine the data significance at p≤0.05 [49]. All statistical analysis was computed with a computer-based COSTAT package (CoHort Software, Berkeley, USA).
RESULTS AND DISCUSSION
In this experiment, Bacillus subtilis (k1) is used as a fermentation organism. Its selection based on that they can produce over to 60% extracellular industrial enzymes [10, 50]. The submerged fermentation cultures of B. subtilis raised on various organs of banana agricultural wastes [51]. The variant richness of cellulose, hemicellulose, and lignin among different parts or organs of banana could have a significant impact on the production of various metabolites to enzymes in the medium. Based on those various banana organs used as a fermentation substrate for B. subtilis growth (Table 1).
The cultures of the B. subtilis were maintained for 12 hours on various banana organ-based cultures. After the incubation period, the OD600 of the culture was read. The cultures showed variant growth rates due to the type of supplemented substrate in the cultures as a carbon source. The lowest growth observed in the LB₁ (control ⅛th concentration of LB₀/TY-medium, table 1), while the maximum cell multiplication rate was recorded in both levels of LB₂ (leaf blade) and LB₅ (floral leaflets) cultures. It was comparatively higher than the full strength LB₀ medium (Table 2). This differential growth rate is due to the carbon fueling levels of the supplemented substrates in the form of different plant organs of banana agro-wastes. An increase in growth rate among all the cultures also noted with the increase in the concentration of the substrates (Table 2).
The cell growth and production of reducing sugars from the supplied agriculture wastes as carbon sources by B. subtilis in submerged fermentation cultures as defined in Table 1. According to the data, the cell biomass production was increased in the cultures than others, while some remained lower. Similarly, variant concentrations of the total sugars and reducing sugars were observed (Table 2). Both cell biomass and sugar concentrations among the cultures have various indications either about the ability of fermentation organisms to grow on the supplied substrate of unable to finish sugars of the medium [52]. After 12 hrs of culture, maximum total sugars found in LB₀ (control), LB₅ (floral leaflets) and LB₆ (fruit peels) than others. It could be possible that filtrates of the floral leaflets and fruit peels in water may be extracted higher sugars after crushing in dH₂O. The reducing sugars were observed higher in LB₆ (fruit peels) and LB₇ (suckers) than other cultures supplemented with banana extracts. Meanwhile, the total proteins are concentrated in LB₄ (leaf sheaths), LB₅ (floral leaflets) and LB₆ (fruit peels).
The maintenance of the fermentation organism in cultures depends on its secretions as well as the production of various substances during the simplification of a supplemented substrate as a carbon source. During the cell culture incubation, an increase in free molecules among cultures like proline and glycine betaine indicates that the organism is under stress from the culture solute load [53, 54] as observed in this study (Table 2). Similarly, the production of phenolics, antioxidants, and flavonoids are also due to stress or could be the secondary products of raised cultures [55–57]. The sources of most of the phenolics, antioxidants, and flavonoids are plants that present in the human diet as their health beneficial and anti-pathogen compounds [58].
However, the scarification rate and enzyme production showed differential data among the plant organ-specific based agriculture waste cultures. The significant variation in enzyme activities has observed among the cultures (Fig 1). The pectinases showed the highest activities in fruit peels than in leaf blades and pseudostem, while amylases were higher in sucker and leaf-midrib supplemented cultures. The cellulases showed maximum activities among the cultures, which are supplemented with leaf blades, fruit peel and leaf sheaths added as the substrate. The lipases have shown maximum activities in the cultures added with leaf-sheath, leaf blade and suckers as fermentation carbon source, while proteases observed higher among the cultures with leaf blades and suckers. Various enzymes are produced with different rates among the cultures. These differential levels of enzymes are specified by the specific composition of plant organs, which are supplemented as carbon sources.
At the harvest time (12 hrs), the presence of pectin, lipids, and starch induces the extracellular production of pectinases, lipases and amylases [52]. Each enzyme leads to hydrolyze their respective substrates in the medium into simple sugars (glucose and maltose), which is utilized by the growing strain [58]. The increase or decrease in the levels of production of enzymes could be managed by the possible proteolytic degradation or its inhibition by the extracellular proteases [52, 58]. Meanwhile, their decomposition or denaturation could occur due to the interactions with other fermented compounds in the medium [59]. The differential levels of proteases indicate the growth stage of the fermentation organism and the availability of nutrients in the medium [60]. For the purpose to fulfill the need for nutrients, the cultured organisms lead to secrete proteases to overcome the deficiency of nutrients from the decomposition of extracellular proteins [60] with its slower growth rates [58].
CONCLUSION
Banana agricultural waste has no doubt numerous quality for the production by Bacillus subtilis (k1). It can be utilized to produce various industrially important enzymes that will ultimately help in the cost-effectiveness and the environment. The above-discussed results mention and show that the banana substrates do have the capability to be replaced with the LB medium mostly the enzyme and biochemical analysis have promoted the 30% concentration of fruit peel, leaf blade, and leaf sheet. Among the entire test, the lipase activity measured was the highest than other enzyme assays. The increase in the substrate concentration might increase the enzyme activity in the case of xylanase and pectinase. The results obtained from the present study could be a reference for important microbial metabolites biosynthesis with banana agriculture wastes.
ACKNOWLEDGEMENT
The authors are very great full to the University of Sindh, Jamshoro for financing the current experiment. Also thankful for using other facilities including the apparatus and especially supporting staff of the laboratory at the institute.
Funding
The study was funded by the University of Sindh, Jamshoro.
Conflict of interest
None.
Table 1: Composition of the various medium used for the cell growth of Bacillus subtilis (k1) supplemented with various plant organs of banana wastes as the substrate
|
#s. |
Medium |
Composition of medium |
|
01. |
LB₀ |
TY-medium (1 % tryptone, 0.5 % yeast extract, 0.5 % NaCl) |
|
02. |
LB₁ |
⅛ LB₀ (nutrient deficit control culture) |
|
03. |
LB₂ |
LB₁ + 15 % leaf blade extract |
|
04. |
LB₂ₐ |
LB₁ + 30 % leaf blade extract |
|
05. |
LB₃ |
LB₁ + 15 % Leaf midrib extract |
|
06. |
LB₃ₐ |
LB₁ + 30 % Leaf midrib extract |
|
07. |
LB₄ |
LB₁ + 15 % Leaf sheaths extract |
|
08. |
LB₄ₐ |
LB₁ + 30 % Leaf sheath extract |
|
09. |
LB₅ |
LB₁ + 15 % floral leaflets extract |
|
10 |
LB₅ₐ |
LB₁ + 30 % floral leaflets extract |
|
11. |
LB₆ |
LB₁ + 15 % fruit peels extract |
|
12. |
LB₆ₐ |
LB₁ + 30 % fruit peels extract |
|
13. |
LB₇ |
LB₁ + 15 % sucker extract |
|
14. |
LB₇ₐ |
LB₁ + 30 % sucker extract |
|
#s. |
Medium |
Culture OD600 |
Total sugars (mg ml⁻¹) |
Reducing sugars (mg ml⁻¹) |
Total proteins (mg ml⁻¹) |
|
|
01. |
LB₀ |
3.610±0.159a |
0.904±0.020a |
0.487±0.017bc |
1.625±0.005ab |
|
|
02. |
LB₁ |
1.503±0.039c |
0.311±0.039i |
0.188±0.012b |
1.254±0.002d |
|
|
03. |
LB₂ |
4.033±0.157de |
0.523±0.010ed |
0.280±0.012b |
1.164±0.017cd |
|
|
04. |
LB₂ₐ |
4.983±0.276ab |
0.651±0.016cd |
0.442±0.041a |
1.406±0.011a |
|
|
05. |
LB₃ |
2.656±0.022c |
0.574±0.006de |
0.232±0.006ef |
1.160±0.002d |
|
|
06. |
LB₃ₐ |
2.558±0.078b |
0.586±0.013de |
0.385±0.020ef |
1.417±0.021b |
|
|
07. |
LB₄ |
3.080±0.143ab |
0.515±0.020fg |
0.280±0.011cd |
1.358±0.006ef |
|
|
08. |
LB₄ₐ |
3.247±0.214de |
0.679±0.012bc |
0.444±0.012ef |
1.382±0.027b |
|
|
09. |
LB₅ |
3.221±0.112c |
0.485±0.011g |
0.280±0.026c |
1.327±0.005de |
|
|
10. |
LB₅ₐ |
3.465±0.270de |
0.720±0.010b |
0.381±0.005cd |
1.451±0.046d |
|
|
11. |
LB₆ |
2.837±0.081d |
0.586±0.012de |
0.444±0.007c |
1.327±0.012de |
|
|
12. |
LB₆ₐ |
3.520±0.035ab |
0.718±0.013b |
0.314±0.001de |
1.305±0.027b |
|
|
13 |
LB₇ |
2.260±0.161bc |
0.394±0.013h |
0.376±0.003c |
1.294±0.024c |
|
|
14. |
LB₇ₐ |
3.409±0.254c |
0.482±0.008g |
0.423±0.016d |
1.429±0.035b |
|
|
Data significance |
24.67*** |
49.02*** |
27.06*** |
33.95*** |
||
|
|
Total phenolics (mg ml⁻¹) |
Ascorbic acid (mg ml⁻¹) |
Free proline (mg ml⁻¹) |
Glycinebetaine (mg ml⁻¹) |
||
|
01. |
LB₀ |
0.021±0.001d |
0.137±0.000c |
0.223±0.005a |
1.457±0.028a |
|
|
02. |
LB₁ |
0.021±0.001d |
0.140±0.000bc |
0.158±0.006d |
0.299±0.006d |
|
|
03. |
LB₂ |
0.017±0.001b |
0.139±0.000cd |
0.165±0.008b |
0.753±0.015c |
|
|
04. |
LB₂ₐ |
0.021±0.001d |
0.142±0.001a |
0.087±0.006ef |
1.263±0.014bc |
|
|
05. |
LB₃ |
0.020±0.000e |
0.136±0.000d |
0.148±0.012ef |
0.397±0.002g |
|
|
06. |
LB₃ₐ |
0.020±0.001d |
0.139±0.000cd |
0.159±0.006c |
0.722±0.008c |
|
|
07. |
LB₄ |
0.017±0.001a |
0.133±0.001de |
0.151±0.003cd |
0.663±0.002de |
|
|
08. |
LB₄ₐ |
0.017±0.001b |
0.141±0.001ab |
0.101±0.002de |
0.309±0.025hi |
|
|
09. |
LB₅ |
0.017±0.001e |
0.137±0.000c |
0.159±0.009c |
0.496±0.002fg |
|
|
10. |
LB₅ₐ |
0.023±0.000cd |
0.141±0.000ab |
0.063±0.004g |
1.286±0.035b |
|
|
11. |
LB₆ |
0.020±0.001c |
0.140±0.001bc |
0.150±0.006d |
0.650±0.047cd |
|
|
12. |
LB₆ₐ |
0.017±0.001cd |
0.142±0.000a |
0.092±0.010ef |
1.458±0.017a |
|
|
13 |
LB₇ |
0.021±0.001b |
0.142±0.000a |
0.068±0.013fg |
0.973±0.007cd |
|
|
14. |
LB₇ₐ |
0.020±0.001f |
0.141±0.000ab |
0.142±0.001cd |
0.620±0.017d |
|
|
Data significance |
10.58*** |
33.56*** |
32.02*** |
11.21*** |
||
|
Note: The data-values are the means of 4-replicates and their standard error, while letters i.e. a,b,c,d … for DMRT and *** for data significant at 0.05 level (5%). |
||||||
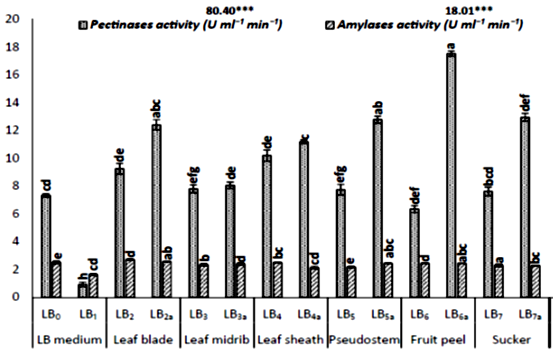
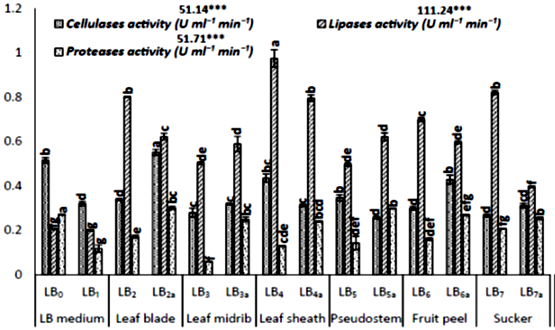
Figure 1: Analysis of different enzyme activities secreted in submerged Bacillus subtilis (k1) cultures supplemented with different organs of banana agriculture waste as substrate. The graphs are the mean values of 4-replicates and their standard error, while letters i.e. a,b,c,d … for DMRT and *** for data significant at 0.05 level (5%).
REFERENCES

