|
Bergenin Exhibits a Nephroprotective Effect by Improvement of the Antioxidant System in Xenobiotic-Induced Oxidative Stress in ICR mice Yollada Sriset1, Sawaros Na Nakorn1, Sukanda Chitsaitarn1, Koranat Dechsri1, Waranya Chatuphonprasert2, Kanokwan Jarukamjorn1* |
|
1 Research Group for Pharmaceutical Activities of Natural Products using Pharmaceutical Biotechnology (PANPB), Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen 40002 Thailand. 2 Faculty of Medicine, Mahasarakham University, Maha Sarakham 44000 Thailand. |
ABSTRACT
Xenobiotic-induced oxidative stress is a principal cause of kidney damage. Antioxidants may prevent oxidative stress that leads to kidney damage. Bergenin, a C-glucoside derivative of gallic acid, is abundant in plants of the Mallotus, Bergenia, and Ardisia genera. Bergenin has been reported as an effective antioxidant that can scavenge free radicals and has previously been shown to protect rat and mouse kidneys against oxidative damage, but its nephroprotective impact against xenobiotic-induced renal oxidative damage has not been assessed in vivo. The present study assessed the nephroprotective effects of bergenin on the renal antioxidant system in ethanol, sodium selenite (SS), and tert-butyl hydroperoxide (TBHP) induced renal oxidative stress in mice. Adult male ICR mice were administered ethanol (1.5-2 g/kg/day; peroral, p.o.), SS (4 mg/kg/day; p.o.), or TBHP (18 mg/kg/day; i.p.) in combination with bergenin (10, 50, and 250 mg/kg/day; p.o.) or gallic acid (100 mg/kg/day; p.o.; positive control) for 7 consecutive days. Ethanol, SS, and TBHP suppressed the activity of the main renal antioxidant enzymes, namely superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Furthermore, ethanol, SS, and TBHP depleted renal glutathione stores, which substantially decreased the reduced glutathione (GSH) to oxidized glutathione (GSSG) ratio, and boosted the formation of renal malondialdehyde (MDA). Treatment with bergenin and gallic acid attenuated the renal damage associated with xenobiotic-induced oxidative stress in the mouse kidneys by increasing the activity of the SOD, CAT, and GPx antioxidant enzymes and restoring the glutathione stores, which lead to an extraordinary decline in MDA levels. Thus, bergenin and gallic acid demonstrated a comparable nephroprotective potential that was mediated through the improvement of the renal antioxidant defense machinery. Therefore, bergenin is a promising nephroprotective candidate for xenobiotic-induced oxidative stress.
Key Words: Antioxidant activity, Gallic acid, Glutathione store, Nephroprotection, Xenobiotic.
INTRODUCTION
Kidneys are vital and highly energetic organs that perform crucial functions including the removal of metabolic by-products through urine, regulation of blood pressure, and maintenance of essential electrolytes/fluid balance. [1-3] Hence, kidneys are susceptible to injury by toxic agents, especially the free radical by-products from drug and xenobiotic metabolism, and damage to the kidneys can result in a homeostatic imbalance in the body. [2, 4] Oxidative stress through overproduction of free radicals leads to progressive deterioration of renal function [5]. As such, oxidative stress is likely to be the main cause of kidney disease of unknown etiology. [6, 7]
Humans are exposed to toxic compounds through diet, environment, and behavior. [8] Xenobiotics such as ethanol, sodium selenite, and tert-butyl hydroperoxide (TBHP) are toxic and cause damage to various organs through the generation of free radicals, principally reactive oxygen species (ROS). [1, 9] Ethanol and sodium selenite may be obtained via beverages, diets, and lifestyle. [10, 11] TBHP is an industrial solvent, and exposure is typical as an environmental pro-oxidant pollutant. [12] The kidneys are a target organ of oxidative stress as they accumulate ROS. [13] Oxidative stress can trigger a vicious cycle of renal pathology that damages biological structures and impairs renal function. [14] Intake of polyphenol-rich natural compounds may protect against renal oxidative stress by scavenging ROS and stimulating the antioxidant system. [1]
Bergenin, a C-glucoside of polyphenol 4-O-methyl gallic acid (Figure 1A), is a natural derivative of gallic acid. [15] It is abundant in plants such as Mallotus japonicas, M. repandus, M. philippinensis, Caesalpinia digyna, Ardisia colorata, Bergenia ciliata, and B. ligulata. [15] Bergenin content was reported in M. repandus stem extract as 19% dry weight [16] and in M. philippinensis stem and root extracts as 4% and 6% dry weight, respectively. [17] Bergenin has been claimed to have antioxidant, [18] hepatoprotective, [19] antidiabetic, [20] anti-inflammatory, [21] and gastroprotective activities. [22] Bergenin contained in B. ciliata and B. ligulate was demonstrated to have effective nephroprotective activity in rat and mouse models. [23, 24] The strong polyphenol antioxidant gallic acid (Figure 1B) is found in extracts of Terminalia chebula and Phyllanthus emblica fruits, Camellia sinensis leaves, and Vitis vinifera seeds (0.7%, 2.4%, 15%, and 16% dry weight, respectively). [25, 26] Gallic acid showed nephroprotective activity against cisplatin and carbon tetrachloride-induced kidney damage in rats. [27, 28]. Gallic acid was therefore employed as a positive standard in our study of the nephroprotective and antioxidant properties of bergenin in ethanol, sodium selenite, and TBHP-induced oxidative stress model in mouse kidneys.
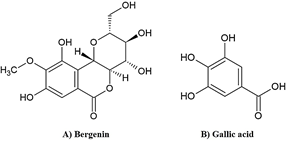
Figure 1: The chemical structures of A) bergenin and B) gallic acid.
MATERIALS AND METHODS
Chemicals
Bergenin (Cat. BP0258, purity > 98%) was a product of Biopurify Phytochemicals (Chengdu, China). Absolute ethanol and gallic acid were obtained from Merck (Darmstadt, Germany). Tert-butyl hydroperoxide (TBHP, 70% w/v), sodium selenite, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), L-glutathione reduced (GSH), L-glutathione oxidized (GSSG), glutathione reductase (GR), 5,5¢-dithiobis (2-nitrobenzoic acid) (DTNB), β-nicotinamide adenine dinucleotide 2′-phosphate (NADPH), 4-vinyl pyridine (4-VP), malondialdehyde (MDA), and thiobarbituric acid (TBA) were products of Sigma-Aldrich Chemical (St. Louis, Missouri, USA). All other laboratory chemicals were of the highest purity and obtained from chemical suppliers.
Animals and experimental design
Male ICR mice (7-weeks-old), weighing 30-35 g, were supplied by Nomura Siam International Co., Ltd., Bangkok, Thailand. All mice were housed in the Animal Unit of Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand, under the controlled temperature of 23±2°C and humidity of 45±2% with a 12 h dark/light cycle. The protocol for animal treatment was approved by the Institutional Animal Ethics Committee for Use and Care of Animals, Khon Kaen University (Approval No. IACUC-KKU-86/61). At all times, the mice were housed on corn cob bedding in stainless steel cages with water and commercial mouse diet supplied ad libitum.
Mice were divided into 16 groups of five mice: control, ethanol-, sodium selenite-, and TBHP-treated groups were orally administered bergenin (10, 50, and 250 mg/kg/day) or a positive control gallic acid (100 mg/kg/day) for 7 consecutive days. The control was simply given distilled water (0.1 mL/mouse/day). For the ethanol-treated group, mice were orally administered ethanol at 2 g/kg/day for the first 3 days followed by ethanol at 1.5 g/kg/day for the last 4 days. For the sodium selenite and TBHP-treated groups, mice were administered sodium selenite (4 mg/kg/day) orally or TBHP (18 mg/kg/day) intraperitoneally for 7 days.
At 24 h after the last treatment, all mice were euthanized using Zoletil® (250 mg of tiletamine and 250 mg of zolazepam, Virbac New Zealand, Hamilton, New Zealand) at a dose of 100 mg/kg. Kidneys were immediately excised and kept at -80°C for further analysis.
Determination of protein content in mouse kidneys
Kidney (1 g) was homogenized in 0.01 M phosphate-buffered saline (PBS, 3 mL) with a hand homogenizer in an ice-bath. Protein content was determined using the Bradford method as previously described. [29] Briefly, 40 µL of diluted kidney homogenate was mixed with 160 µL of the Bio-Rad Protein Assay reagent (Bio-Rad, Hercules, CA, USA). The absorbance of the protein-dye complex was measured at a wavelength of 595 nm and compared with standard bovine serum albumin (0.0125-0.15 mg/mL).
Assessment of superoxide dismutase (SOD) activity in mouse kidneys
Kidney homogenate was mixed with chloroform and ethanol (1:1.67) before centrifugation at 14,000×g at 4°C for 30 min. The supernatant was mixed with a reaction mixture containing 1.1 mM xanthine, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.6 mM nitroblue tetrazolium, 56 mM sodium carbonate, 70 µg/mL bovine serum albumin, and 0.02 mUnits/µL xanthine oxidase and incubated at room temperature for 20 min. After incubation, 0.1 mM copper (II) chloride was added. Absorbance was measured at a wavelength of 550 nm and calculated as SOD activity by comparison with the standard SOD. [30]
Assessment of catalase (CAT) activity in mouse kidneys
Kidney homogenate was incubated with 150 mM hydrogen peroxide (H2O2) at 37°C for 1 min. The reaction was terminated by adding ammonium molybdate at a final concentration of 26 mM, followed by absorbance measurement at a wavelength of 405 nm. CAT activity was calculated by comparison with the standard CAT. [30]
Assessment of total glutathione (total GSH), reduced glutathione (GSH), and oxidized glutathione (GSSG) contents in mouse kidneys
Kidney homogenate was extracted in 5% w/v sulfosalicylic acid (1:5) and subjected to centrifugation at 10,000×g at 4°C for 10 min. The supernatant was mixed with a freshly prepared mixture of 7 mM potassium phosphate buffer (pH 7.0), 0.04 Units/mL GR, and 0.03 mg/mL DTNB, followed by addition of 0.04 mg/mL NADPH. Absorbance was measured at a wavelength of 405 nm at 30 sec-intervals for 10 min. Total GSH content was calculated by comparison with the slope of the standard GSH (6.25-50 µM). [31]
The GSSG content assay was performed by incubation of the supernatant with 4-VP for 60 min at room temperature followed by the addition of the reaction mixture. GSSG content was calculated by comparison with a slope of the GSSG standard curve (5-30 µM) while GSH content was calculated by subtracting GSSG content from total GSH content. [31]
Assessment of glutathione peroxidase (GPx) activity in mouse kidneys
GPx activity was assessed as previously described with some modifications. [30] Kidney homogenate was mixed with a reaction mixture containing 2.5 mM sodium phosphate buffer pH 7.4, 0.01 mM EDTA, and 8.3 µM sodium azide and incubated at 30°C for 10 min. This was followed by the addition of 0.7 mM GSH substrate and 1.2 mM H2O2 to start the reaction. The reaction was terminated by adding 3.3% w/v sulfosalicylic acid before centrifugation at 330×g for 15 min. The supernatant was employed for the assessment of GPx activity as described previously. [31] The GPx activity was defined as the amount of GPx that produces 1 µmol of GSSG per 1 min.
Determination of lipid peroxidation by thiobarbituric acid reactive substances (TBARS) assay in mouse kidneys
Kidney homogenate was mixed with 10% w/v trichloroacetic acid (1:1) followed by centrifugation at 2,300×g at 4°C for 10 min to obtain a supernatant. An equal volume of 0.8% w/v TBA was added to the supernatant before boiling at 100°C for 15 min, followed by immediate cooling in an ice-bath. Finally, MDA was measured by a spectrofluorometer at an excitation wavelength of 520 nm and an emission wavelength of 590 nm by comparison with the MDA standard curve (5-40 µM). [31]
Statistical analysis
The results are expressed as mean±standard deviation (SD) (n=5). Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test using Statistical Package for Social Studies (SPSS) IBM version 22.0 (Armonk, New York). p<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
The cellular antioxidant system is comprised of enzymatic (SOD, CAT, and GPx) and non-enzymatic (glutathione) antioxidant systems that scavenge free radicals to help maintain an oxidant-antioxidant balance. [9] SOD, the first step of the enzymatic antioxidant defense against free radicals, converts superoxide anion radical (O2-) to H2O2, which is subsequently transformed to oxygen and water by CAT and GPx. [1, 7] GSH and GSSG are the main compounds in the glutathione system. Active GSH neutralizes ROS via electron donation. [32] GSH is oxidized into GSSG by GPx and GSSG is returned to GSH by GR. [7, 32] Preservation of the GSH and GSSG balance is critical for maintaining glutathione homeostasis. [9, 32] Therefore, the overwhelming of cellular antioxidant capacity by excessive levels of free radicals is a decisive factor in oxidative stress. [9] In the present study, ethanol, sodium selenite, and TBHP were used to induce oxidative stress in mouse kidneys.
Ethanol significantly decreased SOD (Figure 2), CAT (Figure 3), and GPx (Figure 4) activities in mouse kidneys. Ethanol also extensively diminished total GSH and GSH contents with the simultaneous elevation of GSSG content, which impaired the GSH/GSSG ratio in mouse kidneys (Table 1). Moreover, the production of MDA in mouse kidneys was significantly increased by ethanol (Figure 5).
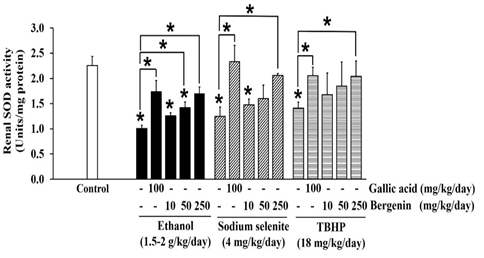
Figure 2: Effects of bergenin on SOD activity in ethanol, sodium selenite, and TBHP-induced oxidative stress in mouse kidneys. The data are presented as mean±SD (n=5). A significant difference was determined by a one-way analysis of variance followed by Tukey’s post hoc test. *p<0.05.
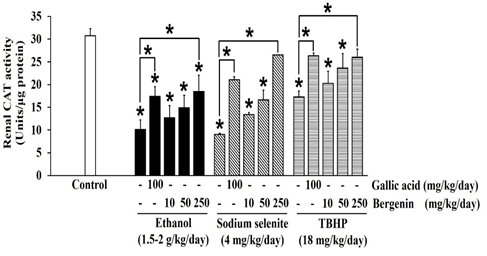
Figure 3: Effects of bergenin on CAT activity in ethanol, sodium selenite, and TBHP-induced oxidative stress in mouse kidneys. The data are presented as mean±SD (n=5). A significant difference was determined by a one-way analysis of variance followed by Tukey’s post hoc test. *p<0.05.
These findings correspond with a previous study that showed an increase in MDA level, decreases in SOD, CAT, and GPx activities, and depletion of GSH content in kidneys of male albino Wistar rats orally administered ethanol (4 g/kg/day) for 4 weeks. [14] Similarly, a daily oral dose of ethanol (5 g/kg/day) for 8 weeks was nephrotoxic in male Wistar rats; serum urea, creatinine, and uric acid levels were heightened with an associated increase in renal MDA level, while renal SOD, CAT, and GPx activities dropped and glutathione stores were depleted. [33] Also, oxidative stress characterized by increased MDA formation and lessening SOD, CAT, and GPx activities was reported in kidneys of male Swiss mice treated with ethanol (2.5 g/kg/day) for 14 days. [34] Taken together, ethanol doses ranging from 2.5 to 5 g/kg/day were nephrotoxic in rats and mice. [14, 33, 34] In our model, we lowered the dose of ethanol (1.5-2 g/kg/day) to mimic the moderate drinking stage of alcoholism. [35] Our observations suggest that ethanol at the lower dose impaired cellular antioxidant defenses and induced oxidative stress in mouse kidneys through the generation of the toxic metabolite acetaldehyde and several ROS, especially O2-, α-hydroxyethyl, and hydroxyl radicals, via the alcohol dehydrogenase pathway. [14, 36]
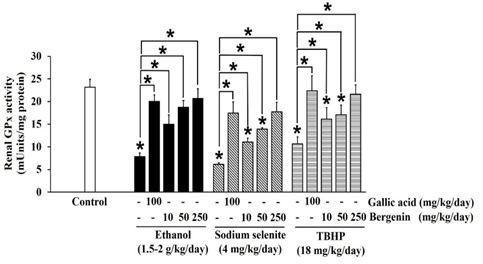
Figure 4: Effects of bergenin on GPx activity in ethanol, sodium selenite, and TBHP-induced oxidative stress in mouse kidneys. The data are presented as mean±SD (n=5). A significant difference was determined by a one-way analysis of variance followed by Tukey’s post hoc test. *p<0.05.
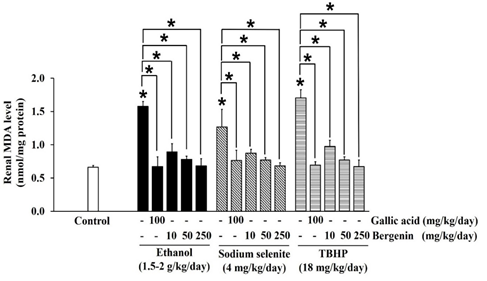
Figure 5: Effects of bergenin on the formation of malondialdehyde (MDA) in ethanol, sodium selenite, and TBHP-induced oxidative stress in mouse kidneys. The data are presented as mean±SD (n=5). A significant difference was determined by a one-way analysis of variance followed by Tukey’s post hoc test. *p<0.05.
Sodium selenite was previously reported as a nephrotoxic agent in a rabbit model, [37] but the dose could not transfer to the induction of oxidative stress in our mouse kidney oxidative stress model. The dose of sodium selenite at 4 mg/kg/day was selected based on previous studies that noted events of oxidative stress and impairment of the antioxidant system in mouse livers. [38, 39] In the present study, sodium selenite induced oxidative stress in the mouse kidney could be seen in significantly reduced SOD (Figure 2), CAT (Figure 3), and GPx (Figure 4) activities and included depletion of glutathione stores via decreases in total GSH and GSH contents with an associated increase in GSSG content, which lead to an extensive reduction in the GSH/GSSG ratio (Table 1). The level of MDA was also significantly elevated in kidneys of mice-treated with sodium selenite (Figure 5). These observations revealed the potential of sodium selenite to induce the oxidative stress pathway in mouse kidney through the glutathione system’s production of ROS by-products, mainly O2-, [10, 40] and the accumulation of selenium, which can also provoke nephrotoxicity. [37]
Table 1: Effects of bergenin on glutathione profiles in ethanol, sodium selenite, and TBHP-induced oxidative stress in mouse kidneys.
|
Treatments |
Glutathione contents (nmol/mg protein) |
The ratio of GSH/GSSG |
|||
|
Total GSH1 |
GSH2 |
GSSG3 |
|||
|
Normal |
Control |
60.32 ± 2.20 |
53.64 ± 2.15 |
6.68 ± 0.25 |
8.03 ± 0.39 |
|
Ethanol induction (1.5-2 g/kg/day) |
Ethanol Gallic acid 100 mg/kg/day Bergenin 10 mg/kg/day 50 mg/kg/day 250 mg/kg/day |
16.21 ± 0.11*
46.43 ± 0.84*$
31.04 ± 3.05*$ 39.80 ± 4.67*$ 44.12 ± 1.77*$ |
4.30 ± 0.59*
38.27 ± 0.76*$
21.97 ± 2.87*$ 31.08 ± 4.44*$ 36.21 ± 1.72*$ |
11.91 ± 0.55*
8.16 ± 0.56*$
9.08 ± 0.23*$ 8.72 ± 0.35*$ 7.92 ± 0.48*$ |
0.36 ± 0.03*
4.69 ± 0.36*$
2.42 ± 0.27*$ 3.56 ± 0.43*$ 4.57 ± 0.36*$ |
|
Sodium selenite induction (4 mg/kg/day) |
Sodium selenite Gallic acid 100 mg/kg/day Bergenin 10 mg/kg/day 50 mg/kg/day 250 mg/kg/day |
18.93 ± 0.73*
47.90 ± 1.40*Ψ
34.31 ± 2.11*Ψ 39.22 ± 2.86*Ψ 46.57 ± 4.33*Ψ |
3.27 ± 0.53*
38.56 ± 1.04*Ψ
22.51 ± 1.99*Ψ 29.26 ± 3.06*Ψ 37.10 ± 4.37*Ψ |
15.66 ± 0.53*
9.34 ± 0.58*Ψ
11.80 ± 0.51*Ψ 9.96 ± 0.65*Ψ 9.47 ± 0.61*Ψ |
0.21 ± 0.03*
4.13 ± 0.23*Ψ
1.91 ± 0.18*Ψ 2.94 ± 0.44*Ψ 3.92 ± 0.52*Ψ |
|
TBHP induction (18 mg/kg/day) |
TBHP Gallic acid 100 mg/kg/day Bergenin 10 mg/kg/day 50 mg/kg/day 250 mg/kg/day |
18.05 ± 0.85*
46.70 ± 1.22*#
31.85 ± 1.16*# 39.68 ± 3.11*# 43.19 ± 1.77*# |
1.75 ± 0.20*
37.79 ± 1.72*#
20.01 ± 1.01*# 29.93 ± 4.16*# 34.16 ± 2.05*# |
16.31 ± 1.50*
8.92 ± 0.85*#
11.84 ± 0.44*# 9.75 ± 1.18*# 9.03 ± 0.35*# |
0.11 ± 0.01*
4.24 ± 0.56*#
1.69 ± 0.10*# 3.07 ± 0.70*# 3.78 ± 0.37*# |
Note. The results are expressed as mean±SD (n=5). 1, total glutathione; 2, reduced glutathione; 3, oxidized glutathione. A significant difference was determined by a one-way analysis of variance followed by Tukey’s post hoc test. *p<0.05 vs Control; $p<0.05 vs Ethanol induction; Ψp<0.05 vs Sodium selenite induction; #p<0.05 vs TBHP induction.
The 18 mg/kg/day dose of TBHP was modified from models of TBHP-induced oxidative damage and toxicity in livers and kidneys of rats and mice. [41, 42] Our results showed that TBHP provoked a significant reduction in SOD (Figure 2), CAT (Figure 3), and GPx (Figure 4) activities and depletion of total GSH and GSH contents with an increase of GSSG content, which subsequently lead to a significant reduction in GSH/GSSG ratio in mouse kidneys, compared with the control (p < 0.05) (Table 1). MDA level was also significantly raised in mouse kidneys treated with TBHP, compared with the control (p < 0.05) (Figure 5). These observations supported induction of renal oxidative stress by TBHP corresponding with a previous study in which decreased antioxidant enzyme activities (SOD, CAT, GPx, and GR) and increased MDA level in rat kidneys were revealed after intraperitoneal administration of TBHP (30 mg/kg/day) for 2 weeks. [41] TBHP-induced renal oxidative damage can be explained by the production of its toxic metabolite tert-butyl alcohol and ROS, mainly peroxyl and alkoxyl radicals, via two distinct pathways in kidneys consisting of cytochrome P450 2E1 and the GPx-GR system. [41, 43, 44]
The ROS generated by ethanol, sodium selenite, and TBHP was responsible for the induction of oxidative stress in the mouse kidneys as evidenced by the decrease in renal antioxidant status. Moreover, metabolism of ethanol, sodium selenite and TBHP generates both toxic metabolites and ROS that can rapidly react with biomolecules such as proteins, lipids, and nucleic acids, which then leads to the impairment of renal structure and function. [2, 45] Kidney cell membranes are rich in long-chain polyunsaturated fatty acids, and ROS directly attacks these fatty acids leading to lipid peroxidation to produce the end-product MDA. [2, 46] This affects the kidney cell membrane’s integrity, leading to membrane fragility and the loss of selective membrane permeability. [47] The present study suggests that ethanol, sodium selenite, and TBHP injured mouse kidneys by accelerating lipid peroxidation along with depression of the antioxidant system. Therefore, preventing damage to the antioxidant system might be a suitable target to attenuate renal oxidative damage.
In our study, bergenin dose-dependently increased SOD (Figure 2) and CAT (Figure 3) activities, and the highest dose of bergenin prevented the reduction of SOD (Figure 2) and CAT (Figure 3) activities by ethanol, sodium selenite, and TBHP-induced oxidative stress in mouse kidneys. A significant elevation of GPx activity was shown by bergenin in a dose-dependent manner in ethanol, sodium selenite, and TBHP-induced oxidative stress in mouse kidneys (Figure 4). Furthermore, bergenin demonstrated a dose-dependent increase in total GSH and GSH contents and a decrease in GSSG content that restored the GSH/GSSG ratio in ethanol, sodium selenite, and TBHP-induced oxidative stress in mouse kidneys (Table 1). Remarkably, bergenin dose-dependently reduced renal MDA levels in mice treated with ethanol, sodium selenite, and TBHP to levels comparable to the control (Figure 5). These findings correlate with a previous study revealing bergenin (10 mg/kg/day) exerted nephroprotective activity in ethylene glycol-induced renal injury in male Sprague Dawley rats via elevation of SOD and CAT activities and improvement of the GSH/GSSG ratio. [23] Similarly, the study of Ambika and Saravanan (2016) reported a nephroprotective effect of bergenin (10-40 mg/kg/day) against oxidative stress damage from high fat-diet in male C57BL/6J mice via a decrease in MDA level and maintenance of antioxidant status (SOD, CAT, and GPx activities and GSH content). [48] Moreover, bergenin (20-100 mg/kg/day) showed the ameliorative effect on high-glucose and high-fat diet-induced renal damage in male C57BL/6 mice through the inhibition of renal oxidative stress and activation of nuclear factor erythroid-derived 2-related factor 2 which up-regulated antioxidant enzyme activities (SOD, CAT, and GPx) in mouse kidneys. [49] Correspondingly, our study showed that gallic acid greatly enhanced SOD (Figure 2), CAT (Figure 3), and GPx (Figure 4) activities, augmented total GSH and GSH contents, and reduced GSSG content resulting in maintenance of the GSH/GSSG ratio (Table 1), and also reduced MDA level (Figure 5) in ethanol, sodium selenite, and TBHP-induced oxidative stress in mouse kidneys. According to a previous study, the nephroprotective effect of gallic acid (50-200 mg/kg/day) against carbon tetrachloride-induced renal damage in female Sprague Dawley rats was demonstrated via recovery of GSH content and inhibition of lipid peroxidation. [28] Finally, gallic acid (20-40 mg/kg/day) was reported to increase antioxidant enzyme activities (SOD and GPx), restore GSH content, and suppress MDA level in cisplatin-induced nephrotoxicity in male albino rats. [27]
According to the present study, both bergenin and gallic acid prevented ethanol, sodium selenite, and TBHP-induced renal oxidative stress in mice by balancing the enzymatic and non-enzymatic antioxidant status. Thus, bergenin demonstrated nephroprotective properties.
CONCLUSION
Ethanol, sodium selenite, and TBHP induced ROS overproduction leading to oxidative stress in mouse kidneys, which was alleviated by bergenin. Bergenin, especially 250 mg/kg/day, was able to protect against renal oxidative damage by reactivating the antioxidant defense system (SOD, CAT, GPx, and glutathione stores) and inhibiting lipid peroxidation. Therefore, the nephroprotective activity of bergenin correlated directly to its antioxidant potential. Bergenin is a suitable candidate for further development as a natural health product for antioxidation and nephroprotection.
ACKNOWLEDGMENTS
This work was supported by Research Group for Pharmaceutical Activities of Natural Products using Pharmaceutical Biotechnology (PANPB2563), Faculty of Pharmaceutical Sciences, Khon Kaen University, Thailand. The authors thank Dr. Glenn Borlace, Khon Kaen University for English language assistance.
REFERENCES

