



|
Antimicrobial Activities of Different Novel Chitosan-Collagen Nanocomposite Films Against Some Bacterial Pathogens Mashail Al-Ghamdi1, Magda M. Aly1*, Reda M. Sheshtawi2 |
|
1 Department of Biology, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia. 2 Department of Chemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia. |
ABSTRACT
Resistance to antibiotics is increased in all hospitals, and bacterial pathogens that contaminate burns and wounds are widespread. Their resistance to well-known antibiotics is recorded, and every day resistant isolates are counted and studied. The present study aimed to prepare new polymers with antimicrobial activities. The effect of some polymers prepared from chitosan and collagen associated with nanoparticles of Cu+, Ti4+, and Ag+ was determined using some bacterial pathogens as test organisms. The tested bacteria were two gram-negative, Pseudomonas aeruginosa and Escherichia coli and four gram-positive, Enterococcus faecalis, Streptococcus sp., Staphylococcus aureus and Methicillin-resistant Staphylococcus aureus [MRSA]. The antimicrobial activity of the prepared polymers was determined using three methods; diffusion method, colony-forming unit [CFU] and Optical density method. The beetroot plant was extracted by boiling water to obtain the water extract which was used to prepare the polymers. The results showed that all nanocomposite films were active against different pathogenic bacteria. The antibacterial activities of the discs of four different prepared films were compared. The most active film was Chitosan-Collagen nanocomposite with three different metals. Gram-negative Pseudomonas aeruginosa and Gram-positive MRSA were the most sensitive bacteria in incorporating films. In conclusion, films of chitosan, collagen, and nanoparticles [CuO, TiO2, AgNO3] had excellent antibacterial abilities against both Gram-positive and negative bacteria. Thus, they can be used as dressing in wounds and burns for healing.
Key Words: Chitosan; Collagen; CuO; TiO2; AgNO3; Antimicrobial activity, Pseudomonas aeruginosa.
INTRODUCTION
Chemotherapeutic materials or antimicrobial agents were discovered and used for treatments of many diseases and it was believed that treatment with these materials leads to the disappearance of infectious diseases. However, many bacterial and fungal pathogens were controlled by antibiotics for years and started returning in new forms, resistant to the antibiotic. These drug-resistant microorganisms caused incidents of epidemics which was a common global problem with public health concerns. The multi-drug resistant bacteria had global emergence which limited the effectiveness of current antibiotics and caused significantly treatment failure. The most resistant Gram-positive bacteria are MRSA, penicillin, and macrolides resistant pneumococci and, vancomycin-resistant enterococci as well as many multidrug-resistant Gram-negative bacteria. Increasing resistance to old antibiotics must be associated with the development of new antibiotics. The resistance problem was continued, thus new antibiotics are needed to stop and disturb resistance mechanisms and increased recovery of the patients in all hospitals.
Chitin was after cellulose in abundance. It has unique biological characteristics and activities. The natural sources of chitin are insect exoskeletons, shells of arthropod (shrimp, prawn, crabs, and cephalopod beaks), and fungal cell walls [1]. Chitosan is a linear polysaccharide derived from the most common natural chitin. Many promising biological activities were recorded for chitosan which mainly processes antimicrobial and antitumor activities in addition to wound healing acceleration [2, 3]. Chitosan is mainly studied due to its interesting properties including nontoxicity, complete biodegradability, and antimicrobial abilities. Chitosan can be used in pharmacy, foods, cosmetic, and textiles industries, agriculture, and treatment of wastewater [4]. Chitosan has a unique capacity to combine strongly with metal ions due to the presence of free amino groups [5, 6]. There are two explanatory models for the structural connection of chitosan to metal ions: the “pendant model”, where only one amino acid group of chitosan is bound to one ion, and the “bridge model”, where several nitrogen atoms, hydroxyl groups or even more than one chitosan chain are all bound to one ion [7, 8].
Similarly, collagen is a natural protein found in human bodies that interacts and regulates cell anchorage, movement, proliferation, and growth [9]. Moreover, collagen can promote tissue regeneration. Collagen has increased importance because it is a promising biopolymer and has medical importance. Chitosan and Collagen have the abilities of film formation with biocompatibility and biodegradability [10].
Sulfanilamide is an antibiotic, form of 4-amino benzene which is mainly used to treat dangerous diseases caused by bacterial infections, especially Beta hemolytic Streptococcus and Staphylococcus aureus [11].
Plant extracts like antibiotics inhibit many bacteria including resistant S. aureus and other gram-negative bacteria. These bacteria prompted antibiotics but the presence of the plant extract acts as MDR Pump or Efflux Pump inhibitors. The multiple drug-resistant isolates of S. aureus resist three or more agents including ciprofloxacin, erythromycin, vancomycin, clindamycin, and gentamicin. Similarly, Mycobacterium tuberculosis resists many currently used drug classes (isoniazid, rifampicin, fluoroquinolones, and aminoglycosides). Thus, new antibiotic sources from plants and their extracts are needed because they have active products that may be inhibitory for bacteria [12].
In the last few decades, looking for new materials with antimicrobial activities from nanocomposite film have growing importance and their results are difficult to compare. Many researchers need methods with accurate results for the development of antibiotics from plants or microorganisms. Therefore, in the present study, biofilm properties of nanocomposite film to ensure were investigated in vitro to discover the resources of new pharmaceutical compounds. This study studied the durability and laundering and inhibitory effects of the different chitosan and collagen nanocomposite films against some resistant bacteria.
MATERIALS AND METHODS
Materials
Chitosan with medium molecular weight is purchased from ACROS ORGANICS Company (Geel, Belgium), Glutaraldehyde, collagen (MW: 500-30000) from fish skin, titanium oxide nanoparticles (TiO2), copper oxide nanoparticles (CuO), and silver nitrate (AgNO3) were purchased from Boss chemical industry C., LTD (Jinan, China)
Extraction of Plant pigment
Fresh roots of Beetroot (25 g) were placed with 500 ml of warm water for 12 h at room temperature. The aqua extract was passed through Whatman filter paper No.1. and put in small vials at 4°C [13]. The absorbent was analyzed using a UV spectrophotometer (Perkin Elmer) at 220-440 nm.
Preparation of the Ag NPs
This solution was freshly prepared by dissolving 0.5 g Silver nitrate in 10 ml dist. water under constant magnetic stirring. Then, 10 ml of beetroot extract solution, A550 = 1.0, was added to the solution under continuous stirring until NPs were formed. The Ag NPs formation was noticed by changing the solution color from red to brown.
Preparation of the TiO2 NPs
TiO2 (0.5 g) was dissolved in 2% wt./v. glacial acetic acid under stirring in 250 ml beaker for 24 hours at room temperature to ensure a homogeneous solution. The homogeneous solution was filtrated through Whatman filter paper no. 0.1 and preserved at room temperature until used.
Preparation of chitosan-collagen-nanocomposite films
The method described by Vargas et al. [14] with some modifications was used for film formation. Chitosan and collagen (1% w/v) were hydrated in an aqueous solution of glacial acetic acid for 10 minutes using a magnetic stirrer at 400 rpm. The chitosan-collagen film was fabricated first with sulfonamide by cross-linking with Glutaraldehyde (GL), 1 % w/v; cross-linked composite film incorporated by beetroot extract. Afterward, 1 ml of TiO2NPs, 1 ml Ag NPs or 0.0025g of CuO NPs were gradually added (10g was homogenized for 5 min at 13000 rpm). Another film was prepared after adding 1 ml of both TiO2 NPs, 1 ml Ag NPs and 0.0025 g of CuO NPs. The resultant films were cast on the center of Petri dish plates and the plates were dried at room temperature for 48 h. Dried film materials were removed and stored at room temperature (20-25°C) until used.
Antimicrobial activities
The tested bacteria, Pseudomonas aeruginosa, Escherichia coli, Enterococcus faecalis, Streptococcus sp., Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) were obtained from the Hospital of King Abdulaziz, Jeddah, Saudi Arabia.
Inhibitory activities of the nanocomposite films were determined using different test microorganisms. The agar disc diffusion technique was applied. Under sterile conditions, Petri plates, each containing 20 ml of Muller Hinton agar were inoculated with 100 µm of the bacterial suspension. Using a sterile cotton swab, the inoculum was spread on the surfaces of agar plates. Using sterile forceps, films of 5mm diameter were put in the agar plate. All plates were kept at 4ºC for one hour, followed by incubation at 37◦C for 24 hours. The obtained zone of inhibition was examined and the mean diameter was calculated.
The surface contact method was used to determine the antimicrobial activities of new sheets or materials. Using this technique, bacteria were applied directly on the surface of the examined material. After incubation, the inhibition was detected and determined.
The disks of films [5 mm diameter] were put in separate wells of a sterile 24-well plate. In each well, 20 µl of the bacterial inoculum was added. After that, the 24-well plate was incubated aerobically at 37 °C for one hour. After incubation, each disk was washed with sterile water and transferred to a 15 ml pre-sterilized tube containing 5 ml of the solution of sterile Phosphate buffer [pH 7.2]. The previous solution composed of NaCl 8g, KCl 0.2g, Na2HPO4 1.44 g, KH2PO4 0.24 g in 1000 ml of distilled water. The PBS buffer with the disk film was vortexed for 1 min at 50 Hz to ensure bacterial removal.
After serial dilutions, 100 µl was put on Muller Hinton Agar plates and the plates were incubated at 37°C for 24 hours. The viable colonies in each sample were expressed [CFU/ml]. Disk films were placed on Muller Hinton Agar to ensure the antibacterial surface film.
The viable bacterial colonies were counted and the inhibition rate percentage for each film was determined:
Inhibition rate [%] = [B – A]/B × 100
B and A are respectively the mean number of bacteria in control and treated samples after 24 hours of incubation.
Bacteria were grown on agar medium at 37°C for 12 hours, then one colony was grown in Muller Hinton Broth medium at 37°C for a night. The bacteria were then seeded on 100 ml Muller Hinton Broth to make the bacterium concentration at 105CFU/ml. 5mm disk films disinfected by UV light were then added. After incubation at 37°C for one day, the absorbance was calculated at 600nm.
The inhibitory percentage was calculated by the following equation:
Growth reduction [%] = OD 6001 - OD 6002 / OD 6001× 100
The four nanocomposite films prepared from the previous experiment were tested by putting these films in a bacterial suspension (4X104 CFU/ml) and incubated for 24 h. Immediately each film was picked up and put on the Muller Hinton Agar plate. All plates were incubated at 37°C for 24 h and the plates were examined and photographed.
Statistical analysis
Statistical analyses were carried out using SPSS version 16 and the result was expressed as mean ± standard deviation (Mean ± SD).
RESULTS
Beetroot plant was collected and extracted using boiling water and the obtained extract was identified using a UV spectrophotometer. The obtained results were analyzed and summarized in Figure 1. The maximum absorption was obtained at 536 nm. The prepared extract was used for silver nitrate reduction (Figure 2 A). The obtained Ag NP was characterized using UV spectra and maximum absorption was obtained at 800 nm (Figure 2 B).
Antimicrobial activity of chitosan-collagen nanocomposite films by diffusion method
Antibacterial activity for chitosan-collagen nanocomposite films was determined using well diffusion agar methods. According to the obtained results, the combination of nanoparticles with chitosan-collagen showed synergistic antimicrobial activity against all tested bacteria (Table 1, Figure 3). Chitosan-collagen with CuO NPs was active against E. coli and S. aureus (diameter of zone of inhibition was 7.0 mm) as shown in Table 1, whereas, chitosan-collagen with TiO2 was active against S. aureus with mean inhibition zone diameter of 7.3±0.10 mm. Chitosan-collagen-Ag NP showed the highest activity against P. aeruginosa followed by S. aureus (17.00±0.12 and 13.30±0.07 mm), respectively, while chitosan-collagen without nanoparticles did not show inhibition zone against all tested bacteria.
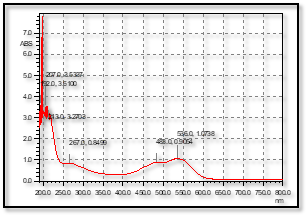
Figure 1: UV-Spectra of diluted Beetroot extract used for the preparation of Ag NPs.

Figure 2: A: The prepared silver nanoparticles using beetroot extract [a: before, b: after Ag NPs preparation], B: UV-Spectra of silver nanoparticles.
Chitosan-collagen CuO, TiO2 and Ag nanoparticle films [Figure 3A] showed excellent inhibition areas against tested bacteria as seen in Table 1. The lower activity of the previous film was observed on Streptococcus sp. with an inhibition zone of 9.00±0.04 mm [Figure 3E] and the highest activity was against P. aeruginosa with inhibition zone o 16.00±0.05 mm as in Figure 3B. Results showed moderate antibacterial activity of the film on S. aureus, MRSA (Figure 3 C & D), E. faecalis and E. coli with inhibition zones of 12.00±0.09, 11.00±0.03, 12.00±0.07, 15.00±0.06 mm, respectively.
Antibacterial Durability and Laundering Durability of chitosan-collagen nanocomposite films by colony-forming unit
The antibacterial activity of chitosan-collagen nanocomposite film was used for E. coli, P. Aeruginosa, S. aureus, MRSA, E. faecalis, and Streptococcus sp. by using Laundering Durability procedure as shown in Figure 4 A & B. The results were presented with the CFU (Figure 5).
Treatment of bacteria by using chitosan-collagen loading with nanoparticles showed a very effective antibacterial activity against all tested bacteria as seen in figure 4 A & B. Notably, the incorporation of nanoparticles into composite films increase the antimicrobial activity as compared to the films just incorporated with one nanoparticle; CuO-TiO2-Ag NPs at composite film gives a 100 % bacterial reduction rate (BR%) against Streptococcus sp. and P. aeruginosa but the film with CuO NPs show lower killing rate (near to 40 %) against E. coli. By using CFU, a higher number of colonies appeared on plates in all tested bacteria than that with no treatment by films. Figures 5A & 6A represent the highest count number of untreated MRSA and P. aeruginosa solutions with films. In contrast, fewer colonies were growing on the chitosan-collagen with CuO and with TiO2 NPs films as seen in Figures 4 and 5. The curve in Figure 4 A & B imply that chitosan-collagen with CuO-TiO2-Ag NPs had superior antibacterial activity, and the inactivation activity was 97.7% for MRSA (Figure 5 Ae), 100% for P. aeruginosa (Figure 5 Be), and 100% for E. coli [data not shown].
Table 1. Antimicrobial activity [mm] of chitosan-collagen nanocomposite films compared to its components using the agar well diffusion method.
|
Tested film Tested bacterium |
Film 5 |
Film 6 |
Film 7 |
Film 8 |
|
Staphylococcus aureus |
7.0± 0.11 |
7.3± 0.10 |
13.3± 0.07 |
12.0± 0.09 |
|
MRSA |
ND |
ND |
10.0± 0.04 |
11.0± 0.03 |
|
Enterococcus faecalis |
ND |
ND |
11.0± 0.00 |
12.0± 0.07 |
|
Streptococcus sp. |
ND |
ND |
8.0± 0.02 |
9.0± 0.04 |
|
Pseudomonas aeruginosa |
ND |
ND |
17.0± 0.12 |
16.0± 0.05 |
|
Escherichia coli |
7.0±0.0 |
ND |
13.1± 0.04 |
15.0± 0.06 |
|
Bacterial index |
11 |
7.3 |
72.3 |
75 |
Data are expressed as mean+SD (n = 3), ND: Not detected, Film 5: chitosan-collagen-sulfa-beetroot-CuO NP; Film 6: chitosan-collagen-sulfa-beetroot-TiO2 NP; Film 7: chitosan-collagen-sulfa-betroot-Ag NP; Film 8: chitosan-collagen-sulfa-beetroot-(CuO,TiO2 and Ag) NPs.
Antimicrobial activity of chitosan-collagen nanocomposite films by using optical density [OD 600nm]
Inhibitory effect of chitosan–collagen nanocomposite film by using optical density (OD600 nm) was estimated to show the effect of tested nanocomposite films against tested bacteria. As shown in Table 2, the inhibitory effect was measured based on the absorbent of treated bacterial suspension with tested films and compared by blank bacterial suspension without treatment by films. The values showed a good percentage for Streptococcus sp. and P. aeruginosa. After no bacterial treatment with films, the OD600 absorbents were high. However, when the bacteria were grown in the presence of films, the absorbents were less compared to control (not treated with any film).
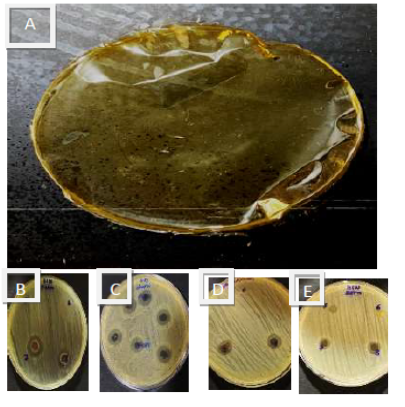
Figure 3: Chitosan collagen nanocomposite film 8 A, the antibacterial activity of the previous film against S. aureus [8 B], P. aeruginosa [8 C] MRSA [ 8D] and Streptococcus sp. [8 E].
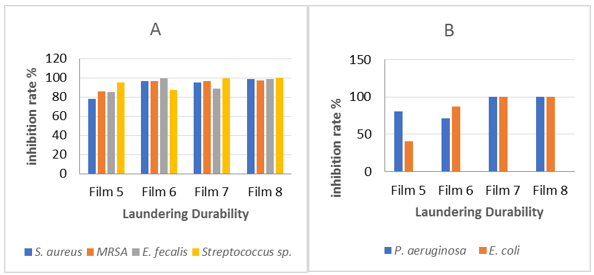
Figure 4: Antibacterial effect of washing durability films against Gram-positive (A) and Gram-negative bacteria (B)
Anti adhesive activity of chitosan-collagen nanocomposite films against two bacterial pathogens, MRSA and S. aureus
The anti-adhesive activity of four prepared films was detected on agar surface plates. The films were immersed in bacterial suspension and incubated for 24 h. Then, the films were picked up and grown on clean plates for 24 h. The anti-adhesive activity of each film was recorded. Figure 6 showed that the chitosan-PVP with Ag NP (Films 7 and 8) showed low or no bacterial growth while the others were surrounded by bacteria. The results for the two bacteria were the same. We can conclude that films with chitosan-PVP with Ag NP or incorporation of three nanoparticles showed excellent antibacterial activities.
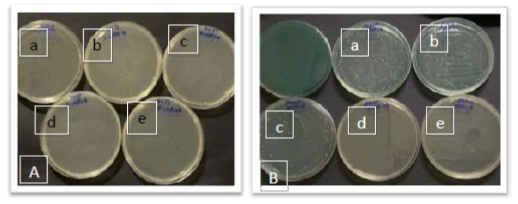
Figure 5: Counts of MRSA [A] and P. aeruginosa [B] after film treatment: from control [no film] [a], chitosan-collagen-sulfa-beetroot-CuO NP Film [b]; chitosan-collagen-sulfa-beetroot-TiO2 NP Film [c]; chitosan-collagen-sulfa-betroot-Ag NP Film [d]; chitosan-collagen-sulfa-beetroot-[CuO,TiO2 and Ag] NPs Film [e].
Table 2. Antimicrobial activity measured by growth inhibition percentage [OD 600 nm] of chitosan-collagen nanocomposite films.
|
Tested films |
S. aureus |
MRSA |
E. faecalis |
Streptococcus sp. |
P. aeruginosa |
E. coli |
|
Film 5 |
76.50% |
84.22% |
83.13% |
92.61% |
75.79% |
37.40% |
|
Film 6 |
94.85% |
94.82% |
96.88% |
84.68% |
68.39% |
83.94% |
|
Film 7 |
93.66% |
95.01% |
86.13% |
96.70% |
100% |
96.94% |
|
Film 8 |
96.81% |
95.73% |
96.2% |
98.00% |
100% |
96.89% |
Film 5: chitosan-collagen-sulfa-beetroot-CuO NP; 6: chitosan-collagen-sulfa-beetroot-TiO2 NP; 7: chitosan-collagen-sulfa-beetroot-Ag NP; 8: chitosan-collagen-sulfa-beetroot-[CuO, TiO2, Ag] NPs.
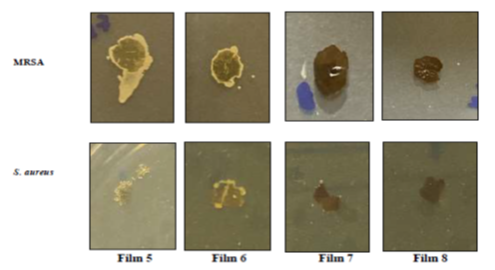
Figure 6. The anti-adhesive activity of chitosan-collagen nanocomposite films against two bacterial pathogens, MRSA and S. aureus. Film 5: Chitosan-collagen-sulfa-beetroot-CuO NP; 6: Chitosan-collagen-sulfa-beetroot-TiO2 NP; 7: Chitosan-collagen-sulfa-beetroot-Ag NP; 8: Chitosan-collagen-sulfa-beetroot-[CuO, TiO2, Ag] NPS.
DISCUSSION:
In this work, chitosan-collagen nanocomposite film was prepared with three different nanoparticles (CuO, TiO2, and Ag) and beetroot plant extract as described by Vimala et al. [15] method with some modifications. They also found the chitosan-Polyvinyl alcohol Ag NPs had a good antimicrobial ability against E. coli, P. aeruginosa, and S. aureus.
Chitosan and collagen have higher antibacterial activity due to interaction between the positive charge on amino groups [NH] which has a unique capacity to combine strongly with metal ions on the bacterial negatively charged cell membrane, and can disrupt the cell wall of bacteria leading to cell damage [16, 17]. Moreover, the addition of Ag NPs to chitosan/collagen solution demonstrably increased the antimicrobial activity of the film.
Ag NPs have a small size that can penetrate the bacterial cell wall, denaturant cell permeability which leads to cell death. Another probable mechanism of Ag NPs is by contacts of Ag+ with several important enzymes.
The nanocomposite film with Ag and with the incorporation of CuO, TiO2 and Ag NPs had higher antibacterial activity than the nanocomposite with one of NPs. Chitosan-collagen nanocomposite films were tested against bacteria, using three different methods. In the diffusion method, the inhibition zone was only showed when chitosan-collagen incorporated with nanoparticles, which could be due to the stability of polymers and no solubility in media where nanoparticles can diffuse on media. A previous study produced ZnO/Chitosan bionanocomposite films for packaging poultry meat and showed antibacterial activity against foodborne pathogens S. aureus and E. coli [18].
As shown in Table 2, using the CFU [Laundering durability] method, our result shows the mortality range between 40.4%-100%. Moreover, Valizadeh et al. [2019] have shown that the chitosan carboxymethyl cellulose film was active against P. aeruginosa and Listeria monocytogenes and the colony-forming unit was enhanced [19]. Applerot et al. [2012] and Yalcinkaya and Lubasova [2017] measured the effect of CuO NPs. They found that the smallest CuO NPs size, the highest inhibitory activity [20, 21].
By using optical density [OD] of 600nm, our results showed that P. aeruginosa and Streptococcus sp. were the bacteria that gave a high percentage of inhibitory rate by 100 % and 98 %, respectively, as bacterial growth was decreased by ZnO-chitosan nanocomposite film and the antimicrobial activity [22].
Films of Chitosan-PVP with Ag NP or with the three tested nanoparticles had excellent anti-adhesive activities measured by the bacterial growth on the tested films. Similarly, Zhu et al. [23] measured the anti-adhesive activity of chitosan and its derivative and reported that they had high anti-adhesive activities and were non-toxic which made them promising anti-adhesive materials.
CONCLUSION
The present study illustrates a new method in producing novel chitosan-collagen nanocomposite films. The incorporation of three different nanoparticles with two polymers has shown great antimicrobial activity. Further, this study explains a promising application in wound healing and food packaging.
REFERENCES