




Lactic acid bacteria (LAB) effects are related to producing anti-microbial agents as bacteriocin. Our target is to isolate LAB from different horse milk samples and investigates its antimicrobial activities against many pathogenic bacteria. LAB was isolated from horse milk from Al-Taif city in Saudi Arabia on MRS medium. By agar well diffusion method, twelve LAB isolates demonstrated that the antibacterial activities against Gram-positive bacteria S. aureus ATCCBAA977, ST. pneumonia ATCC49619, and E. coli ATCC35218, P. aeruginosa ATCC27853. According to the 16S rDNA sequence, the highest activity LAB isolate was presented in a gene bank such as Lactobacillus plantarum with an identity of 99% under accessory LBP 135401.1. The results showed that the incubation at 35°C for 24h at pH 6.5 were the best conditions for producing the largest quantity of bacteriocin.
INTRODUCTION
Bacteriocins are proteinaceous products produce by many Gram bacteria either positive or negative and have antimicrobial activities [1-4]. Bacteriocins are usually not called antibiotics to avoid anxiety about curative antibiotics could be unlawful allergic effects on humans health [5].
Bacteriocin is protein agents digest through digestive proteases and peptides make from ribosomes, which could improve its properties to enhance its activities [6]. Bacteriocins also can inhibit foodborne pathogens [7, 8]. Bacteriocins products by LAB are advantageous on survival rates of bacteria growth rates in the ecological niche and could exploit by the food industry for example to controlling undesirable bacteria [9]. Lactobacilli has gain attention related to its bacteriocins products [10].
LAB is nonpathogenic, nonsporulating, rod or coccus-shaped, and Gram-positive and is one of the commensal microorganisms found in gastrointestin, urogenit tracts, and milk maternal [11]. LAB was classified as bacteria majority (probiotics) [12, 13]. The LAB group is currently categorized in Firmicutes [14]. Various antimicrobials produce by probiotics, bacteria are effective molecules that subject to intense studies [15].
MATERIALS AND METHODS
Samples
For isolating LAB, 10 samples of Hores milk were used in this research (nearly 150 ml) of Horse milk were collecting from different regions at Al-Taif city in Saudi Arabia.
LAB isolation
Dilution pour plate method uses to obtain LAB isolates from the milk samples (10-4). 0.1 ml of dilution transfer over solid MRS plates. Bacterial colonies were obtained after 24h (incubating / 35°C). Selected colonies were purified and cultured in slants and preserved at 4°C for other studies. Morphological properties of colonies (color, shape, and size) were examined, and morphological properties by using microscopic.
Test bacteria
For ATCC bacteria were used as test organisms: S. aureus ATCCBAA977ST, pneumonia ATCC49619, E. coli ATCC35218, P. aeruginosa ATCC27853. The test bacteria were obtained from IMC in Jeddah, Saudi Arabia.
Screening for production bacteriocin from LAB isolates by agar well diffusion assay
For the study of bacteriocin products and antibacterial activity in the cell-free filtrate (CFF). The cultural filtrate of the bacterial isolate experiments performed in 250 ml Erlenmeyer flasks had 25 MRS ml broth medium and inoculated by slant from preculture of selected isolate (Lactobacillus plantarum) at 35°C. post 24 h of incubation, CFF collecting throughout centrifugation at 5000 rpm / 20 min /4°C. Bacteriocin activities evaluation using agar-well diffusion [16]. 0.1ml of inoculum suspension pours and spread by sterile swap. Wells made by sterile cork borer and fill by supernatant (100 µl). Left plates for 30 min in refrigerator. Plates incubated at 35°C /24 h.
Molecular characterization of selected LAB isolates
Genomic DNA isolation by Gene JET Genomic DNA Purification Kit-Thermo, Fisher Scientific. An amplicon of 1500 bp fragment represented full-length 16S rRNA gene was amplified using highly conserved universal primers [17]. Primers designed for PCR amplification:
Universal primers forward: (27F) 5'AGAGTTTGATCCTGGCTCAG-3'
and reverse: (1492R) 5'TACGGYTACCTTGTTACGACTT-3'
DNA sequences were phylogenetically analyzed and compared with those of GenBank to check for close evolutionary relatives using the BLAST algorithm and RDP database.
Influences of growth conditions for bacteriocin producing by Lactobacillus plantarum H7 isolate
100 μl CFF from cultures place in 5-mm diameter wells and cut in agar plate seed with indicator bacteria. Plates were incubating at 35°C. Post 24 h diameter of inhibition of indicator measure triplicate time for each treatment.
Incubation temperatures effects
Culture broth of Lactobacillus plantarum H7 isolate was incubated for 24 h. at different temperatures (25, 30, 35, 40, and 45 ºC).
Incubation periods of effects
The influence of incubation periods was studied by incubating the culture broth of Lactobacillus plantarum H7 isolate at 35ºC, for different incubation periods (24, 48, and 72 h), the antibacterial activities measure.
PH effects
Different pH (4.5, 5.5, 6.5, 7.5, and 8.5) were used and incubated at 35°C. post 24 h. of incubation bacterial growth and antibacterial activities measured
Statistical analysis
Analyses data were used by SPSS, version 16. Significance of differences among samples determined by t-test. P>0.05.
RESULTS AND DISCUSSION
LAB isolating
Ten samples of fresh horse milk from several places in Jeddah city, in Saudi Arabia. Using MRS agar medium, twelve bacteria isolates were obtained from horse milk. The cultural characteristics of the bacterial isolates were obtained from different milk samples. All the cultural characteristics of the bacterial colonies were smooth surface, colony shape round, flat, and color characteristics white and morphological properties showed gram-positive isolate.
Screening and estimation of antimicrobial activity of LAB isolates by agar well diffusion assay against indicator bacteria
All the obtained LAB isolate screen for produce antibacterial against four different ATCC pathogenic bacteria by agar well diffusion assay. The anti-microbial activities of those were estimating throughout mean inhibition zone diameters (Figures 1 and 2). The mean diameters of inhibition zones were ranged from (15.5-26mm) after 24h of incubation The highest antibacterial activity was by isolates H7 against E. coli ATCC35218. The inhibition zone was (26 mm).
|
|
|
Figure 1. Screening and estimation of antimicrobial activities of LAB |
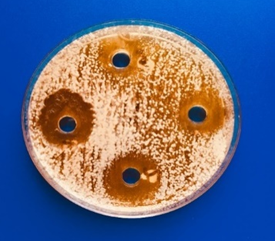 |
|
 |
|
Horse milk isolate against E. coli ATCC35218 |
Horse milk isolate Against St. Pneumonia ATCC49619 |
Horse milk isolate against S. aureus ATCCBAA977 |
|
Figure 2. Production of bacteriocin(s) by LAB |
||
Molecular characterization
Based on the trimmed and merged 16S rDNA sequences comparison analysis for selected isolates by of nearest species sequences retrieve by NCBI BLAST where taxonomic affiliation with isolated strain. DNA sequences analyzed using nucleotides blast alignment tools of gene bank and showed isolated and identified as Lactobacillus Plantarum, with similarity percentages 99 %. The partial 16S rDNA sequence of the selected isolate was submitted into the Bacterial or Archaeal 16S ribosomal RNA sequences database under the accession numbers: LR135401.1 for strain H7 isolated from Horse milk (Table 1).
Table 1. Identity percentage of 16S rDNA of H7
|
Bacterial isolates |
Name and Accession of No. of the most related strain in NCBI GenBank. |
Identity |
Coverage |
Suggested Name and Accession No. of the isolates obtained in the work. |
||
|
H7_27F |
LR135401.1 |
Lactobacillus plantarum |
99% |
99% |
LR135401.1 |
Lactobacillus plantarum |
Cultural conditions influence bacteriocin productions by Lactobacillus plantarum H7 isolate
The various culture conditions were tested to study the optimal conditions on bacteriocin production by the selected isolate.
Incubation temperature effects on bacteriocin(s) productions by Lactobacillus plantarum H7 isolate
Results of this test in Figure 3 show that the highest antibacterial activity against all indicator bacteria when isolate cultures incubating on 35ºC, the range of the inhibition zone varied from 23-25mm [18-22]. The highest antibacterial activity was 25mm against E. coli ATCC35218 and 24mm against S. aureus ATCCBAA977 and ST. Pneumonia ATCC49619 respectively.
Incubation period Effects on bacteriocin(s) productions by Lactobacillus plantarum H7 isolate
Results in Figure 4 show that the highest antibacterial activity against all indicator bacteria when the selected isolate was incubating (24 h). The range of the inhibition zone varied from 24-26mm. The highest antibacterial activity 26mm against E. coli ATCC35218. Increasing the incubation periods decreased the antibacterial activity of the selected isolate.
pH effects on bacteriocin productions by Lactobacillus plantarum H7 isolate
The highest antibacterial activity by Lactobacillus plantarum H7 which was grown at pH6.5 against all indicator bacteria after 24h of incubation at 35º and lower the pH value the production of the antibacterial activity decreased. The highest antibacterial activity was 26mm against E. coli ATCC35218 (Figure 5).
|
|
|
Figure 3. Incubation temperature effect on bacteriocin(s) productions by Lactobacillus plantarum H7 |
|
|
|
Figure 4. Effect of incubation period on the production of bacteriocin(s) by Lactobacillus plantarum H7 isolates |
|
|
|
Figure 5. pH Effects on bacteriocin(s) productions by Lactobacillus plantarum H7 isolates |
Many wild strains of LAB are prospective bacteriocins producers [23] and probiotics [24]. Six horse milk samples from Al-Taif city, kingdom Saudi Arabia, were used to isolates LAB bacterial isolates using MRS agar [25]. Our data approved LAB dominated samples microbial flora: twelve LAB strains were isolated from Horse milk. MRS is the best media for isolating LAB [26]. MRS medium is the best culture media for LAB isolating capable of producing bacteriocin [27]. Agar diffusion assay quantifies the abilities of antibiotics to inhibit the growth of bacteri [28].
In our present work, the indicator Gram-positive bacteria were, S. aureus ATCCBAA977, ST. pneumonia ATCC49619. While Gram-negative bacteria were E. coli ATCC35218 and P. aeruginosa ATCC27853. Most of the isolated lactic acid bacterial strains were had a good inhibitory toward Gram-positive and Gram-negative pathogenic indicator strains. Several investigations focus on LAB-secreted antibacterial products that can inhibit unwanted poultry disease pathogens such as E. coli [29, 30], Staphylococcus [31], a stronger antimicrobial property against positive bacteria such as S. aureus [32, 33].
We found the inhibition zones diameter varied between 23 to 25 mm. Schillinger and Lucke (1989) [34] found inhibition scored as positive if the width of clear zone around colonies of producer strain 0.5 mm or more. Our obtained results showed that LAB isolates were inhibited the growth of the Gram-positive and Gram-negative indicator bacteria, which might be related to bacteriocins productions and peptides with bactericidal activities against those strains of related species [35]. Also Danial et al. (2016) [36] found that the inhibition zone was screened by agar well diffusion method of forty isolates of lactic acid bacterial strains was ranged from 24.00 to 18.33 mm against E. coli ATCC and S. aureus ATCC25923 respectively.
Molecular identification of LAB, using DNA sequencing of 16S, and according to Nocker et al. (2004), [37] amplification 16S sequences of bacteria can produce 750bp amplicon. The DNA sequences analyzed using Blast alignment tools of GenBank and showed that one isolate was identified as Lactobacillus plantarum H7 with 99%. 16S rRNA gene, molecular marker, was ubiquitous to members of this domain [38].
The suitability of environmental conditions (incubation period, temperature, pH, and different media) on the productivity of the production of bacteriocin was investigated. Temperature is also required for growth and bacteriocin production, the highest antimicrobial activity at the optimum growth temperature of 35ᴼC followed by 30ᴼC. Sarika et al. (2010) [39] reported that bacteriocin activity recorded maximum in MRS medium at 30ºC compared to the other incubation temperatures (20, 25, 35, and 40ºC). According to Kumar et al. (2012) [40], the temperature was effective for bacteriocin production by L. casei LA-1 ranging from 33.5 to 34.5°C. These results are incompatible with Barman et al. (2018) [41].
Danial et al. (2016) [36] recorded the best conditions for bacteriocin production by Leuconostoc mesentroides in static incubation culture at 35°C for 24 hr. and pH 6.2. The isolate showed optimal inhibition activity against the tested bacteria after 24 and 48h of incubation. The antagonistic activity of isolates was decreased after 72 h of incubation. In general, the three isolates demonstrated optimal inhibition activity against the tested bacteria after 24 and 72 h of incubation [42]. Also Kp et al. (2016) [43] found protein concentration higher after 48 h of incubation than post 24 and 72 h from incubating.
The highest inhibition zone was recorded at pH 6.5 at 35ᴼC after 24h of incubation. The antibacterial agents were stable within variable pH ranges from 4.5 to 8.5. The inhibition effects were more obvious in the range of pH values (5.5 to 7.5). Also Kumar et al. (2012) [40] reported that pH (6.8 to 7.2) has an effect on bacteriocin productions by L. casei LA-1.
CONCLUSION
Bacteriocins have been used as biological preservatives for about 20 years. Many studies focused on inhibiting food spoilage and human pathogenic bacteria growth by using bacteriocins instead of chemical preservatives and antibiotics, which produced by many living organisms, especially many animals raw milk. In this study, we were able to produce and purify the bacteriocins produced by the lactic acid bacteria isolated from horse milk and improve the conditions such as temperatures, acidity, incubation period and the use of residues that affect the production.
Acknowledgments: None
Conflict of interest: None
Financial support: None
Ethics statement: None