



|
An Overview on Trigeminal Neuralgia, Background, Diagnosis, and Pharmacological Treatment
Mohammed Awad Al Qahtani1, Mohammed Naif Aljuraysi2, Ahmed Abdullah A Abu Alsaud3, Abdullah Oudah Al jabal4, Omar Mohammed Ali Alfaifi4, Bader Abdulaziz Alsehali5, Nada Faiez Alshanbari6, Suhayb Taher Shafy6, Abdullah Mohammed AlJifri7, Sultan Ammash Alsubaie8*, Rwan Ahmed Alshammari9 |
|
1Emergency Department, Ahad Rufaidah general hospital, Khamis Mushait, Saudi Arabia 2 Faculty of Medicine, Northern Border University, Arar, Saudi Arabia 3 Internal Medicine Department, Qatif Central Hospital, Qatif, Saudi Arabia 4 Faculty of Medicine, King Khalid University, Abha, Saudi Arabia 5 Internal Medicine Department, Aliman General Hospital, Riyadh, Saudi Arabia 6 Internal Medicine Department, King Abdulaziz Hospital, Jeddah, Saudi Arabia 7 Faculty of Medicine, Umm Al Qura University, Makkah, Saudi Arabia 8 Faculty of Medicine, Almaarefa University, Riyadh, Saudi Arabia. 9 MD, Neurology Department, Adan Hospital, Kuwait |
ABSTRACT
Introduction: Trigeminal neuralgia is a neurological condition defined as severe, unilateral facial electric-like pain that originates from one or more branches of the fifths nerve, typically the maxillary and/or the mandibular nerve. Pain occurs suddenly in paroxysms and lasts for a few seconds to two minutes. Pain can be intolerable and affect a patient's daily function and quality of life, including eating, drinking, or shaving. The condition is commonly caused by vascular compression of the fifth nerve entry zone, but it can be related to other neurological diseases such as multiple sclerosis or occipital lobe tumors. Objective: We aimed to search for clinical characteristics, possible etiologies, diagnostic tests, and pharmacological treatment of trigeminal neuralgia. Method: We searched in the PubMed database looking for relevant articles, and using the Mesh term "trigeminal neuralgia". Conclusion: Trigeminal neuralgia causes excruciating facial pain that might disrupt the patient's life. Diagnosis is achieved clinically, but brain imaging must be included to rule out the potential secondary cause. The first-line pharmacological treatment is carbamazepine and oxcarbazepine. Other anti-epileptic drugs and botulinum toxin-A injection can be used in addition to the classic regimen, especially if pain persists or side effects are unbearable.
Key Words: Trigeminal neuralgia, diagnosis, management approach
INTRODUCTION
Trigeminal neuralgia (TGN) is first described in the second century by Aerates of Cappadocia, a contemporary of Galen [1]. He described the pain as 'spasm and distortion of the countenance take place' [1]. Jujani, an Arab physician in the 11th-century, reports unilateral facial pain provoking spasm and anxiety [1]. Interestingly, he recommends that the pain is caused by 'the proximity of the artery to the nerve' [1]. In 1773, Hohn Fothergill published a full description of TGN to the Medical Society of London [1]. He defined the typical pain of TGN, including paroxysmal unilateral facial pain, elicited by eating, speaking, or touch with an abrupt onset and ending, and correlated with anxiety [1]. TGN is defined as a sudden, severe unilateral paroxysmal facial pain, stabbing in nature, and usually described by patients as "the world's worst pain” [2, 3]. Pain involves the distribution of one or more branches of the trigeminal nerve, typically, the maxillary of the mandibular nerve Figure 1 [2, 3]. Pain occurs in sudden onset and lasts from a few seconds to 2 minutes, it occasionally becomes unaffordable and prevents drinking or chewing [2, 3]. Some patients are under continuous fear that the pain could abruptly return at any time, and this fear can seriously impair a patient's daily function and reduce the quality of life [4]. The severity of pain can affect daily life measures, including well-being, sleep, mood, and overall health status [4]. TGN causes severe facial pain, which poorly responds to painkillers, mainly if it remains unrecognized [3]. Bilateral trigeminal neuralgia is uncommon, except for secondary trigeminal neuralgia in multiple sclerosis (MS) [5]. The paroxysms frequency range from a few to hundreds of attacks per day, and remission phases can stay for months to years, with a tendency to shorten over time [2]. Conversely, it is also known as 'tic douloureux' [5].
The incidence rate of TGN is estimated to be 4-13 per 100000 people each year [3]. Women are affected twice than men [3]. The incidence gradually increases with age and is uncommon below 40 [3]. The prevalence is 0.07% in the general population, and 2% in patients with facial pain [5]. The diagnosis of TGN is clinically based on characteristics and description of the pain [3, 5]. Therefore, a detailed history is essential for diagnosing, and identifying pain characteristics is essential to establish the diagnosis and treatment [4, 5]. Diagnosis of TGN is insufficient because variant phenotypes must be accounted for, such as typical versus atypical, primary "idiopathic" versus secondary to a significant neurologic disease [5]. In some cases, the pain might emerge from one category to another, like in a typical TGN case, the patients might develop atypical signs later [1]. Oppositely, the pain of TGN might lack its classical characteristics at the beginning, and later developing all hallmarks signs of TGN [1]. Patients with atypical symptoms are more likely to have secondary rather than a primary disease, and they commonly have disease refractory to treatment compared to the classic trigeminal neuralgia [3].
Primary trigeminal neuralgia is infrequently used by many authors to differentiate trigeminal neuralgia with unknown cause and trigeminal neuralgia secondary to neurovascular disease [5]. Hence, the International Classification of Headache Disorders (ICHD) name primary "Classic" trigeminal neuralgia when no identifiable cause other than neurovascular contact is apparent [5]. Around 11% of patients diagnosed with TGN remain without a diagnosis of an exact cause [5]. Based on the International Headache Society (IHS) definition, TGN "may develop without apparent cause or be a result of another diagnosed disorder. There may or may not be, additionally, persistent background facial pain of moderate intensity. Classic TGN develops with no apparent cause other than neurovascular compression." [5].
INTRODUCTION
Trigeminal neuralgia (TGN) is first described in the second century by Aerates of Cappadocia, a contemporary of Galen [1]. He described the pain as 'spasm and distortion of the countenance take place' [1]. Jujani, an Arab physician in the 11th-century, reports unilateral facial pain provoking spasm and anxiety [1]. Interestingly, he recommends that the pain is caused by 'the proximity of the artery to the nerve' [1]. In 1773, Hohn Fothergill published a full description of TGN to the Medical Society of London [1]. He defined the typical pain of TGN, including paroxysmal unilateral facial pain, elicited by eating, speaking, or touch with an abrupt onset and ending, and correlated with anxiety [1]. TGN is defined as a sudden, severe unilateral paroxysmal facial pain, stabbing in nature, and usually described by patients as "the world's worst pain” [2, 3]. Pain involves the distribution of one or more branches of the trigeminal nerve, typically, the maxillary of the mandibular nerve Figure 1 [2, 3]. Pain occurs in sudden onset and lasts from a few seconds to 2 minutes, it occasionally becomes unaffordable and prevents drinking or chewing [2, 3]. Some patients are under continuous fear that the pain could abruptly return at any time, and this fear can seriously impair a patient's daily function and reduce the quality of life [4]. The severity of pain can affect daily life measures, including well-being, sleep, mood, and overall health status [4]. TGN causes severe facial pain, which poorly responds to painkillers, mainly if it remains unrecognized [3]. Bilateral trigeminal neuralgia is uncommon, except for secondary trigeminal neuralgia in multiple sclerosis (MS) [5]. The paroxysms frequency range from a few to hundreds of attacks per day, and remission phases can stay for months to years, with a tendency to shorten over time [2]. Conversely, it is also known as 'tic douloureux' [5].
The incidence rate of TGN is estimated to be 4-13 per 100000 people each year [3]. Women are affected twice than men [3]. The incidence gradually increases with age and is uncommon below 40 [3]. The prevalence is 0.07% in the general population, and 2% in patients with facial pain [5]. The diagnosis of TGN is clinically based on characteristics and description of the pain [3, 5]. Therefore, a detailed history is essential for diagnosing, and identifying pain characteristics is essential to establish the diagnosis and treatment [4, 5]. Diagnosis of TGN is insufficient because variant phenotypes must be accounted for, such as typical versus atypical, primary "idiopathic" versus secondary to a significant neurologic disease [5]. In some cases, the pain might emerge from one category to another, like in a typical TGN case, the patients might develop atypical signs later [1]. Oppositely, the pain of TGN might lack its classical characteristics at the beginning, and later developing all hallmarks signs of TGN [1]. Patients with atypical symptoms are more likely to have secondary rather than a primary disease, and they commonly have disease refractory to treatment compared to the classic trigeminal neuralgia [3].
Primary trigeminal neuralgia is infrequently used by many authors to differentiate trigeminal neuralgia with unknown cause and trigeminal neuralgia secondary to neurovascular disease [5]. Hence, the International Classification of Headache Disorders (ICHD) name primary "Classic" trigeminal neuralgia when no identifiable cause other than neurovascular contact is apparent [5]. Around 11% of patients diagnosed with TGN remain without a diagnosis of an exact cause [5]. Based on the International Headache Society (IHS) definition, TGN "may develop without apparent cause or be a result of another diagnosed disorder. There may or may not be, additionally, persistent background facial pain of moderate intensity. Classic TGN develops with no apparent cause other than neurovascular compression." [5].
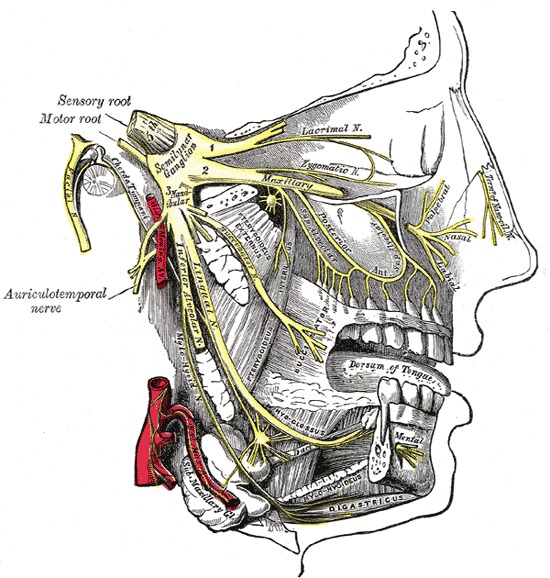
Figure 1. The Trigeminal Nerve, Distribution of the mandibular and maxillary nerves; the submaxillary ganglion. Copied from Gray's Anatomy Book.
DISCUSSION
Trigeminal neuralgia is most commonly caused by trigeminal nerve root compression within a few millimeters of entry into the pons [6]. The nerve impingement is usually associated with demyelination of sensory fibers within the root entry zone or the nerve root, or less frequently in the brainstem [6]. Idiopathic (Primary) TGN is 80-90% caused by vascular compression by a loop of an artery or a vein [6]. Idiopathic TGN leads to morphological alterations in the trigeminal nerve root, and extensive diagnostic investigations may fail to identify the cause [7]. Secondary TGN is caused by structural abnormalities, which affect the trigeminal nerve other than vascular compression, including skull base deformities, multiple sclerosis plaques, or nerve compression caused by benign tumors of the cerebellopontine angle fossa, such as acoustic neuroma, meningioma, and epidermoid cyst [6-8]. The International Association of the Study of Pain (IASP) classification of TGN distinguished TGN caused by MS as a primary and secondary TGN by structural lesions and damages [1]. Secondary TGN accounts for 15% of patients with the disease [7].
Patients with MS have a 20 fold increased risk of developing TGN [7, 9]. Almost 1.9-4.9% of MS patients develop trigeminal neuropathic pain regardless of relapsing-remitting, primary and secondary forms [7, 10]. In contrast, MS is diagnosed in 2-14% of patients with TGN [7, 11]. TGN can be the first presentation of MS in a small number of patients [1]. Those populations are younger than the TGN population, and neuralgia is usually bilateral [1]. MS must be considered in a young patient with TGN, and further workup should be performed to rule out MS [1]. The pathophysiology behind TGN in MS patients is the demyelination involvement of the trigeminal nerve entry zone in the pons [1, 12].
As discussed above, TGN is clinically diagnosed by recognizing the unique features of the trigeminal neuralgia pain. All patients with TGN must have magnetic resonance imaging (MRI) with three high-resolution sequences to identify the cause (excluding vascular compression) [8, 13, 14]. MRI is frequently used in MS patients with TGN [7]. T2-weighted MRI scan is used in MS to identify linear plaques in the ventrolateral pons between the trigeminal nuclei and the trigeminal root entry zone [7]. Since MRI is successfully used to diagnosed primary or secondary TGN, Meany et al. produced a unique MRI technique to recognize the relationship of the impinged nerve and the blood vessels (Magnetic Resonance Tomographic Angiography, MRTA) [1]. Arteries are easily detected in MRTA, but veins are adequately identified only after i.v. gadolinium enhancement [1]. They study the efficacy of this new technique in 55 patients with symptomatic trigeminal neuropathy, and 50/55 were confirmed to have neurovascular contact after posterior fossa exploration of 52 patients [1]. There were no false-positive MRTA and two false negative [1]. The result matches a specificity and sensitivity of 96% and 100%, respectively [1]. To date, no clinical trials are comparing different facial neuropathic pain groups and healthy controls under assessment of blinded radiologists to evaluate the efficacy of MRTA [1]. Therefore, the accuracy of MRTA to differentiate TGN from other forms of facial neuropathic pain remains unknown, and MRTA cannot be used in the diagnosis of TGN [1].
Electrophysiological testing is also used to diagnose TGN [4, 7, 8]. Various neurophysiological techniques can evaluate the trigeminal nerve, and testing the trigeminal reflex has a sensitivity and specificity close to 90% for trigeminal pathway impairment in secondary TGN [7]. Five studies have evaluated the accuracy of electrophysiological testing in distinguishing primary versus secondary TGN [13]. One study used the prospective design, and the remaining wither used case-control design or retrospective data collection [13]. These studies conclude the diagnostic accuracy of trigeminal reflexes for diagnosing secondary TGN, and the result showed a sensitivity of 59% to 100% and specificity of 93% to 100% [13]. Trigeminal reflexes are recommended if MRI is unavailable or contraindicated [14]. An abnormal TGN evoked potential are likely associated with secondary TGN [14]. For all patients with facial neuropathic pain conditions, evoked potentials and trigeminal reflexes are required in the detection of trigeminal nerve afferent damage [14].
Pharmacological treatment is the first-line therapy for TGN if no other structural causes are found [13]. The introduction of carbamazepine in the 1960s markedly replaced the treatment option for TGN, which had been almost exclusively surgical treatment [13].
In acute exacerbations of TGN, management consists of anti-epileptic titration and infusion of intravenous lidocaine or fosphenytoin [14]. For long-term treatment, if the first-line treatment showed failure in TGN or poor adherence, other alternative options can be added or used as a monotherapy, such as lamotrigine, gabapentin, botulinum toxins type A, baclofen, phenytoin, and pregabalin [14-16].
CONCLUSION
Trigeminal neuralgia causes severe unilateral facial shock-like pain that can impair the patient’s daily life and activities. It is a well-known condition since the second century and affecting women more than men. The pain comes in paroxysms and is usually triggered by cheek muscle movement causing the firing of the fifth nerve, such as chewing, drinking, or even talking. The diagnosis is mainly made by taking a good history and clinical description of the pain. Meanwhile, MRI must be considered in all patients with trigeminal neuralgia to exclude possible secondary causes, such as multiple sclerosis or cerebellopontine angle tumors. Classic or primary trigeminal neuralgia is named when no causes are found other than vascular compression, and secondary when nerve entrapment is caused by other neurological conditions, such as demyelination plaques or benign tumors. The mainstay of treatment by sodium channel blockers; carbamazepine and oxcarbazepine. Alternative treatment might be considered when side effects are developed or poor outcome therapy. Many alternative anti-epileptic drugs can be used as monotherapy or in addition to the standard regimen. Studies recommended those alternatives therapy are with some limitations. Therefore, further clinical investigations are strongly needed to establish their efficacy and safety profile.
REFERENCES