|
The Effects of Salicylic Acid on Certain Physiological Parameters in Oryza sativa L. under NaCl Salt Stress
Seyedeh Elham Farhangjoo1*, Ramazan Ali Khavari-Nejad2, Sara Saadatmand1, Farzaneh Najafi3, Babak Babakhani4 |
|
1 Department of Biology, Faculty of Basic Sciences, Islamic Azad University, Science and Research Branch, Tehran, Iran. 2 Professor of Biology, Faculty of Science, Islamic Azad University, Science and Research Branch, Tehran, Iran. 3 Associate Professor of Biology, Department of Plant Sciences, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran. 4 Associate Professor, Department of Biology, Faculty of Basic Sciences, Islamic Azad University, Tonekabon Branch, Tonekabon, Iran. |
ABSTRACT
NaCl salinity stress is one of the most important factors that limits the production of agricultural products. In order to study the effect of using salicylic acid on morphological and biochemical properties of Oryza sativa L. (Neda cultivar) under NaCl salt stress, a factorial experiment was performed in a completely randomized block design with 3 replications. The seedlings were treated with sodium chloride (0, 25, 50, 75 and 100 mM) and salicylic acid (0, 0.5 and 1 mM). The results showed that salicylic acid significantly (P <0.05) reduced morphological traits (germination percentage, plumule and radicle length) under stress conditions and reduced the side effects of salinity on the plants. Also, with increasing concentration of NaCl, proline and malondialdehyde contents and antioxidant activity increased, while adding salicylic acid to the culture medium of plants under NaCl salinity malondialdehyde content reduced and the growth parameters increased. In plants with seeds pre-treated with salicylic acid with increasing proline content, antioxidant activity reduced the side effects of NaCl salinity stress on rice.
Key Words: Salinity Stress, Salicylic Acid, Proline, Malondialdehyde, Antioxidant Enzymes.
Rice (Oryza sativa L.) is an important cereal that is consumed by more than half of the world's population as a main food. The yield of crops is severely affected and reduced by the negative impacts of biotic environmental stresses such as insects, bacteria, fungi, viruses and abiotic stresses such as salinity, drought, heavy metals and pesticides. In order to ensure food security in rice consumer countries, rice production should be increased by 50% by 2025 [1], so it is very important to increase plant resistance to stress in order to produce healthy and useful food.
NaCl salinity is one of the most important environmental factors limiting agricultural products [2]. Salinity has significant effects on all morphological, physiological, biochemical and anatomical traits of most plants, and negatively affects growth, development, survival and production of plants, and the components of treated plant depending on time when the stress was introduced on plant [3]. The main problem of NaCl salinity for higher plants in arid and irrigated lands is the presence of excessive amounts of sodium chloride in the soil [4] because high sodium concentration in the soil reduces the osmotic potential of the soil solution, and the osmotic adjustment mechanism in the plant cells prevents dehydration and difference in the absorption and transfer of ions such as calcium and potassium. High concentration of sodium and chloride ions can have direct toxic effects on membranes and enzymatic systems [5]. Accordingly, sodium and chlorine are two key important ions with ionic effects that significantly reduce the growth and yield of crops [6]. The biochemical changes that occur in environmental stress such as salinity stress produce various active oxygen species, including superoxide (O⁻), hydroxyl (OH⁻), hydrogen peroxide (H2O2) leading to major degradation of membrane, protein, fat and nucleic acids [7]. The plants use a defense system, including an enzymatic and non-enzymatic antioxidant system to combat cell damage. The enzyme's antioxidant system includes catalase, superoxide dismutase, ascorbate peroxidase, and peroxidases [8]. Under normal conditions, there is an equilibrium between the amount of active oxygen species (AOS) production, and the activity of mechanisms for the removal of active oxygen species lost during biotic and abiotic environmental stresses, causing oxidative stress in plants [9]. In addition to oxidative damage through active oxygen species, salinity stress increases some proteins including thermal shock proteins, chaperones and other proteins [10]. Salinity disrupts the absorption of minerals which, by interfering with ion carriers and channels in the root, such as selective K⁺ channels (sodium-potassium competition) and growth inhibition through sodium osmosis, reduces the absorption of water and minerals [3]. One of the natural adjustors is ortho-hydroxybenzoic acid (C7H6O3) or salicylic acid (SA) which belongs to the phenolic compounds group, and is derived from Salix [11]. Salicylic acid is produced by root cells and plays a central role in regulating physiological processes such as growth, ion absorption, photosynthesis and germination in plants [12]. Salicylic acid has been known as a key messenger in activating plant-specific responses. This matter is an important intermediate molecule for plants’ reaction against environmental stresses [13].
The external use of salicylic acid changes the activity of antioxidant enzymes and, with reducing the production of ROS, increases the tolerance of plants to abiotic stresses. Salicylic acid has different effects on plants’ adaptation to stresses, which depends on the concentration and time of use [14]. Study of the effect of salicylic acid on seedlings (seeds’ seedlings) of pistachio showed that salicylic acid reduced the damage caused by salinity stress by preventing the reduction of chlorophyll content, relative humidity, ion leakage and proline content [15]. It has been reported that salicylic acid increased the activity of antioxidant enzymes such as catalase, ascorbate peroxidase and superoxide dismutase, and thus increased the resistance of Brassica juncea against salinity stress [16]. Salicylic acid also reduces the effect of environmental stresses through increasing growth regulating hormones, such as auxins and cytokinins [17]. Given that salinity stress is one of the main challenges of farmers that influences a wide range of plants’ processes, on the other hand, the effects of salinity stress differ on different plants and reduce the plant growth, this study was conducted to investigate the use of salicylic acid under salinity and non-salinity conditions on Neda cultivar and its appropriate concentration for reducing the effects of stress.
MATERIALS AND METHODS
In order to study the effect of salicylic acid pre-treatment on germination, growth, proline content, antioxidant enzymes activities and membrane lipid oxidation of rice seedlings of Neda cultivar under salinity stress conditions, a factorial experiment was performed in a completely randomized design with 3 replications in Tehran Science and Research University physiology lab. Neda cultivar seeds were washed well by 5% sodium hypochlorite for 5 minutes and 96% ethanol for 30 seconds, and were soaked for 24 hours in salicylic acid at concentrations of 0, 0.5 and 1 mM. Subsequently, the seeds were transferred into sterile petri dishes containing filter paper .The salinity treatments were five levels of 0, 25, 50, 75 and 100 mM NaCl.
Inside each petri dish, there was a wet filter paper, where 25 seeds were placed, then petri was sealed with para-film, and kept in a germinator at 25 °C for ten days. The number of germinated seeds was counted daily, the traits of ten day old seedlings were measured.
The plumule and radicle length of the seedlings was measured using a ruler in millimeter. In order to measure the activity of enzymes and determine the peroxidation of lipids and proline of leaves of rice plants, they were stored in a freezer, and kept until the biochemical analysis at -80 °C.
Pigment assay: 0.5 g of fresh leaves were grounded in a porcelain mortar with 15 ml of acetone 80%, and then centrifuged for 10 minutes at 4000 rpm. Absorption of the extract was read by UV / Vis spectrophotometer at 663, 645, and 470 nm wavelengths. For device adjustment, acetone 80% was used. Chlorophylls contents per gram of fresh weight were calculated [18, 19].
Proline assay: 0.5 g of fresh frozen texture were grounded with sulfosalicylic acid 3%. The leaf proline was measured using ninhydrinic acid in accordance with the method provided by Bates et al. (1973) through spectrophotometer at 520 nm [20].
MDA assay: Peroxidation of membrane lipids was measured based on the formation of malondialdehyde complex with Thiobarbituric acid. Malondialdehyde content was measured using Heath and Pacher (1968) method at two wavelengths of 532 and 600 nm using spectrophotometry [21].
Enzymatic extraction
Catalase activity (Fresh frozen texture 0.2 g) was assayed by measuring the initial rate of hydrogen peroxide disappearance [22]. The reaction mixture contained 2.5 ml of 50 mM potassium phosphate buffer (pH 7), 30 mM hydrogen peroxide, and 0.3 ml of enzyme extract. The homogenate was centrifuged at 15000 g for 15 min at 4 ºC, and the supernatant was immediately used for the enzyme assay. Immediately after adding the enzyme extract, the reduction in the absorbance was recorded by 240 nm for 2 minutes and once every 30 seconds by spectrophotometry.
The enzyme activity was expressed in different treatments based on the analysis of 1 mol hydrogen peroxide per minute.
Ascorbate peroxidase (APX) activity was determined by the method of [23]. Frozen Samples of 0.2 g were homogenized in 1 mL of 50 mM potassium buffer (pH 7) containing 0.1 mM EDTA, and 0.5 mM ascorbic acid (ASA), 0.1 mM hydrogen peroxide and 0.1 ml of enzyme extract. The reaction was initiated by adding H2O2to the solution. The decrease in absorbance was monitored at 290 nm.
Data analysis was performed by using statistical software SAS. The mean comparison was done using Duncan's test at a probability level of 5%.
RESULTS AND DISCUSSION
The effect of sodium chloride and salicylic acid on germination percentage
Figure 1 shows that with increasing salt concentration in the media, seed germination percentage reduced, so that it was statistically significant at 5% probability level. While germination percentage of salicylic acid treated seeds increased in saline medium. The highest germination percentage was observed in 0.5 mM salicylic acid without salinity stress and / or 25 mM salinity stress. The lowest germination percentage had a concentration of 100 mM sodium chloride, but no significant difference was observed between the two concentrations of salicylic acid. The germination of seeds that were pre-treated with 0.5 mg salicylic acid was better than that of other stress treatments. As shown in Figure 1, an increase in salicylic acid 1 mM had an inhibitory effect on germination of 25 and 50 m M NaCl .
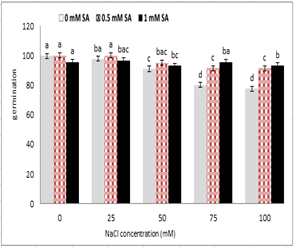
Figure 1: Effect of sodium chloride and salicylic acid on germination percentage of seeds of rice
Seed is one phase of the plant's life cycle which is protected against environmental stresses. After the absorption of water, the beginning of germination, and the onset of growth, the sensitivity to stress conditions is increased [24]. The accumulation of toxic salt ions in the root causes damage to the root system, the metabolism of the plant, growth and product production [25]. Salinity is the ability to disable some hormones, and also the effect on cell membrane permeability reduces vitality. So, in severe stresses, salt induces sleep in many seeds and prevents germination without causing harm to the seeds [3]. The seed germination, especially during exposure to environmental stress, is one of the most critical stages of the plant life [26]. Salinity and drought stress affect regulators such as gibberellic acid, auxin, abscisic acid and changes in the activity of enzymes metabolizing germination carbohydrates. Also, salinity stress increases the content of abscisic acid hormone in the seeds, and thereby prevents germination [27]. According to this research, the use at low temperature, compounds such as salicylic acid, 2, 6 in hydroxy benzoic acid stimulate germination, and also it has been argued that salicylic acid stimulates the germination of carrot plants at low temperatures [28]. In application of salicylic acid on beans and tomatoes, the resistance increases to non-biological stresses such as salinity, drought, cold [29]. This threshold and appropriate concentration of treatment can vary from one cultivar to another and in different plants. In this regard, Shakirova et al. (2003) report showed that due to the effects of salicylic acid at various concentrations, it had a different effect and, with increasing levels, had a positive effect, and hence had a negligible effect on growth [17]. Rahman et al. (2008) in studying the effects of sodium chloride on bread wheat cultivars stated that the increased salinity reduced germination percentage, which would delay the onset of germination [30]. The effect of various concentrations of sodium chloride on germination of two wheat cultivars showed that increasing salt concentration reduced the percentage and the rate of germination, and increased the number of abnormal seedlings [31]. The use of salicylic acid increased the percentage of germination. In summary, the desired effects of treated seeds with salicylic acid on germination included: 1. Increasing the metabolism of proteins and RNA in salicylic acid treated seeds [32]; 2. Increasing the activity of enzymes such as esterase, phosphatase and phosphoglyceride dehydrogenase, which caused the metabolism of storage materials such as carbohydrates in the fetus, lipids, proteins that occurred with the osmotic preparation of the seeds, and finally increased the germination; and 3. Increasing protein synthesis in the fetus, which also led to the increased germination [33]. Salicylic acid improved the germination by increasing levels of auxin and cytokinin in seeds, which stimulated the germination [34]. Also, the treatment of tomato and bean seeds with salicylic acid increased the resistance to stress such as salinity and drought [29].
The effect of sodium chloride and salicylic acid on plumule and radicle length
With increasing salinity, plumule and radicle length reduced in seedlings under salinity stress. The highest plumule and radicle length was observed in the control treatment, and the lowest level was observed at 100 mM sodium chloride level. The use of salicylic acid in comparison to its non-use increased plumule and radicle length in non-stressed treatments. However, no significant difference was observed between the two concentrations of salicylic acid. However, in seedlings under salinity stress, the concentration of 0.5 mM ASA in all treatments of sodium chloride, other than 75 mM, increased plumule and radicle length (Fig. 2 and 3). The seed treatment with salicylic acid at 0.5 and 1 mM had the highest and lowest stem length; respectively, so that from 0 to 0.5 mM salicylic acid, the stem size increased and then reduced (Fig. 2). Regarding the interaction between salinity and salicylic acid, the highest plumule length was observed with 0.5 ml of salicylic acid under control, and the lowest plumule length was observed at 100 mM sodium chloride levels, and non-use of salicylic acid (Fig. 3).
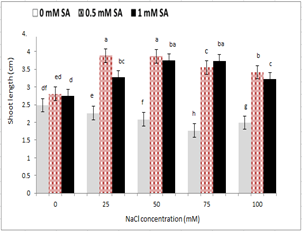
Figure 2 - The effect of sodium chloride and salicylic acid on shoot length of rice seedling
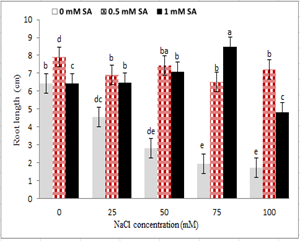
Figure 3: Interaction effect of sodium chloride and salicylic acid on root length of rice seedling
Salt stress also acted like many non-biological stresses which can limit plant growth. Stopping length of the stem, root and the reduction of matter is one of the usual signs of oxidative stress [27, 35]. The storage of metabolic energy can be the basis of growth decline under salinity situation. In this condition, adjusting energy was increasing for ionic and osmotic status while there was a decline in growth energy [36]. Salinity, which affected the penetration of the membrane, cell division, as well as the production of protein and enzymatic activity, could decrease longitudinal growth of plumule and radicle length [37].
As shown in Figures 2 and 3, salinity stress reduced the growth of rice, and the application of salicylic acid together with salinity stress improved the vegetative traits. Reduced growth is a consistency for the survival of plants against all kinds of tensions [38]. Under salinity stress, due to a reduction in water potential surrounding the seed, it took a longer time for the seed to supply its water. Longer germination inhibited plumule and radicle length [39]. The accumulation of sodium and changes in the ratio of K⁺ / Na⁺ in the cytoplasm is effective on the bio-energetic processes. Replacing sodium by potassium can deactivate enzymes, and reduce the growth and even death of cells or plants [40]. The seed treatment with salicylic acid, the concentrations of 0.5 and 1 mM, respectively had the highest and lowest plumule and radicle length (Fig. 2 and 3). Salinity stress by reducing cell growth, cell division and cell size at vegetative growth stage reduced the growth and height of the plant. It has also been suggested that salicylic acid would increase the cell division in barley meristem and improve the plant growth [41]. Salicylic acid effect increasing the rate of using seed reserve increased plumule and radicle length [42]. In chamomile, salinity stress reduced plumule and radicle length, and in treatment with salicylic acid stem length was increased under salinity stress [43]. Similar results have been reported by Yildirim et al (2008) [44] for cucumber, mustard [16], wheat [45] that salicylic acid had a positive effect on root and stem growth parameters, which was consistent with the present study.
Salicylic acid increased the plant height. ABA / IAA ratio has been a very important index of tolerance to salinity stress. This ratio was higher under salinity conditions, salicylic acid probably reduced ABA / IAA ratio significantly. It seemed that the low ratio of ABA / IAA was due to the balance between the two hormones regulated by salicylic acid, which increased the growth under stress conditions [46]. Salicylic acid in wheat exogenously inhibited a reduction in auxin and cytokinin at different levels of salinity, and thus divided the cysts into root meristems, and increased the yield of plants [11]. Salicylic acid increased the growth of root cells which through division, the spread of meristem cells of the primate of the roots increased and finally led to an increase in root length growth [17]. It seemed that seedling dry weight increased due to the increase in plumule, and radicle length was affected by salicylic acid because salinity stress reduced cell division [17]. Also, elements such as sodium and cadmium reduced the growth caused by the reduction in cell division and prolongation of cells by affecting and suppressing the proton pumps [47]. Salicylic acid played an important role in the synthesis of certain proteins such as kinase, which played an important role in regulating cell division, differentiation and morphogenesis [47].
The effect of sodium chloride and salicylic acid on leaf chlorophyll
Chlorophyll a and b contents reduced with increasing salinity concentration, so that the lowest was for 100 mM sodium chloride, and the highest was observed in the control, while in salicylic acid treatment, the highest and lowest chlorophylls contents were for 0.5 mM and 1 mM; respectively (Fig. 4). The highest chlorophyll a was observed in 0.5 mM salicylic acid with 25 mM salinity, and its lowest was at the concentration of 100 mM NaCl, whereas at higher concentrations ( 75 and 100 mM ) of sodium chloride and salicylic acid 1mM increased chlorophyll a content . The highest chlorophyll b was in 0.5 mM salicylic acid with 75 mM NaCl, and its lowest was at 100 mM NaCl (Fig. 4).
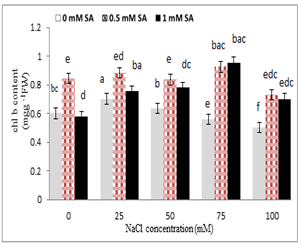
Figure 4. Effect of sodium chloride and salicylic acid on chlorophyll a and chlorophyll b contents in rice seedling
Carotenoids
As shown in Fig. 5, salinity stress caused a significant reduction in the carotenoids of leaves, but the application of salicylic acid increased carotenoids level of leaves. High levels of salinity (75 and 100 mM) reduced carotenoids of leaf, but the combined application of salicylic acid increased the Level carotenoid of leaves. In addition, salicylic acid (0.5 mM) and salinity stress (50, 75 mM) increased leaf carotenoids significantly.
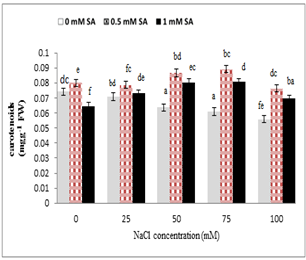
Figure 5. Effect of sodium chloride and salicylic acid on carotenoids contents leaves in rice seedlings
Photosynthesis and cell growth have been among the first processes damaged by salinity and drought stress, which have been directly affected by the limitation of gaseous emissions through mesophilic apertures, changes in photosynthetic metabolism or oxidative stress [48]. The results of the present study indicated that the concentration of chlorophyll a and chlorophyll b significantly reduced in rice under salt stress. So, this reduction was directly correlated with increasing salt concentration of the nutrient solution. Salinity stress increased the concentration of growth regulators, including abscisic acid and ethylene, which stimulated chlorophyllase, thus breaking chlorophylls [49]. Since salicylic acid inhibited ethylene synthesis, it was involved in the improvement of some of the processes involved in the production of chlorophyll [50]. Yildirim et al (2008) stated that chlorophyll content of cucumber was significantly reduced by sodium chloride treatment, but the use of salicylic acid increased the relative proportion of chlorophyll leaves [44]. Tari et al. (2002) reported that the levels of chlorophyll and carotenoids reduced under salinity stress conditions in tomatoes [51]. Salinity stress disrupted the absorption of certain essential elements such as iron and magnesium, which have been essential elements in the synthesis of chlorophyll [52]. Reducing carotenoid content under stress conditions was due to the decomposition of betacarotene and formation of zeaxanthin in xanthophyll cycle [53]. Carotenoids were carried out through the mechanism known as xanthophyll cycle and subsequently epoxidation and dep-oxidation reactions that took oxygen and protected chlorophyll against photovasia [54]. The induction of carotenoid synthesis under stress conditions can be due to their protective role in photosynthetic systems. Because carotenoids caused loss of oxygen alone, and prevented oxidative stress [55]. Stress can degrade chloroplasts and reduced chlorophyll content [56]. Therefore, a reduction in chlorophyll content by stimulation of chlorophyllase was due to the increased concentration of growth regulators such as ABS and ethylene under stress conditions [49]. Salicylic acid prevented ACC synthase activity and ethylene formation, and subsequently reduced chlorophyll content [57].
The role of salicylic acid in increasing leaf chlorophyll was due to increased cytokinin, which played a role in differentiating chloroplast, preventing the destruction of chlorophylls and the bioactivity of chlorophylls [15]. Also, Khodary (2004) stated that salicylic acid spray on maize leaf increased pigments under salinity stress [58]. According to the present study, the amount of photosynthetic pigments reduced under salt stress (Figs. 4 and 5). However, in 100 mM sodium chloride treatment, chlorophyll and carotenoids significantly reduced compared to the lower concentrations. Also, under salinity stress and salicylic acid hormone, a positive effect of salicylic acid on photosynthetic pigmentation was observed. Similar to the results of this experiment, salicylic acid in spinach [59], chickpea [60] and tomato [51] increased carotenoids and chlorophylls contents.
The effect of sodium chloride and salicylic acid on proline content
Treating plants with sodium chloride significantly increased the leaf proline content, so that the lowest proline content in non-stress condition (control) and the highest proline content were observed under 100 mM salinity stress conditions. The mean comparison showed that the highest increase in proline content was in 1 mM SA, and the lowest content was in the control treatment. According to the results of salicylic acid interaction under salinity stress, at each level of sodium chloride, with increasing salicylic acid concentration, proline content significantly increased. This increase was higher in leaf at concentrations of 75 and 100 mM sodium chloride, and the lowest proline content was in the control plants .
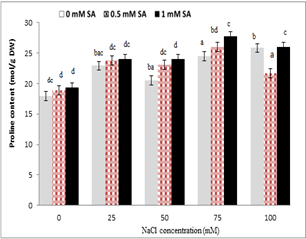
Figure 6. The interaction of sodium chloride and salicylic acid on proline content in rice shoot
Biochemical studies have shown that in the plants under stress, low molecular weight materials, which are called Solution Materials, accumulate [61]. One of the important compounds that respond to saline stress is proline, and its production on the plants that are more resistant to stress. High levels of proline in plants under stress of salinity allow the cells to balance osmotic stresses of their cytoplasm and prevent water shortages [62]. Proline accumulation is one of the mechanisms of plants against salinity stress [63]. Proline played an antioxidant role in cells under stress and, by accumulation in the cytoplasm of the cells, regulated the accumulation of salt in vacuole by reducing the intracellular osmotic potential [64]. Proline accumulation in the plants were stored by two biological pathways, glutamate-dependent pathway, and ornithine-dependent pathway. The glutamate-dependent pathway appeared to be dominant in non-residual stress conditions such as drought, salinity, and the relative importance of ornithine pathway in plants treated with stress depended on the type of species, organ, physical type, and developmental stage of the plant [65]. In this study, the highest proline content was observed under severe salinity stress of 100 mM sodium chloride, which was statistically different from proline content at other salinity levels. Also, the use of salicylic acid under both non-saline and saline conditions proline content increased compared to the control treatment (Fig. 6). This result was consistent with the study results of Gholami and Rahemi (2009) on peach-almond hybrid, and Dehghan et al. (2013) on strawberries that only high levels of salinity stress increased proline content [66, 67]. There have been different reports that a positive correlation was found between proline and Na⁺ concentration under salinity stress, which seemed that proline played the role of sweeping ROS and acting as an antioxidant, and enhancing cellular synthesis during the plant stress [68]. It has been reported that in plants such as wheat and tomato, oxidative stress has increased proline content accumulated with the use of salicylic acid, which increased the resistance of plants to water loss, increasing the content of the shoot as a result of the accelerated growth of plants under stress conditions [69]. It seemed that an increase in proline was due to the osmotic protective role in plants helping membrane stability and reducing negative impacts of sodium chloride on cell membrane degradation. Various reports have indicated that proline content in Ocimum basilicum L. significantly increased under salinity stress, which indicated the production of osmolite and activation of the resistance system of plants against damages caused by salinity stress in the plant [70]. The results of the researchers on tobacco confirmed that the use of salicylic acid has increased proline content in the plant to tolerate the drought [71], which confirmed the peresent study results. One of the reasons for increasing proline content is probably the increase in ABA, which induces proline production. Salicylic acid may also reduce the damage caused by salinity in the plant [17] by inducing the synthesis of intermediate compounds such as ABA, and prevent reducing cytokinin and auxin under drought stress resulting in reduced plant growth [72].
The effect of sodium chloride and salicylic acid on lipid peroxidation
Measuring malondialdehyde content as the final product of membrane lipid peroxidation showed that with increasing stress, malondialdehyde levels also increased, so that the lowest levels of malondialdehyde were under the controlled conditions (without stress), and the highest were under severe stress conditions of 100 mM. Study of the interaction between salinity stress and salicylic acid showed that at the highest stress level (100 mM sodium chloride), plants treated with 0.5 and 1 mM salicylic acid reduced the content of malondialdehyde in the shoot compared to the control plants. The effect of salicylic acid and salinity levels (100 and 75 mM) of sodium chloride, concentration of 1 mM salicylic acid, with a significant reduction of malondialdehyde content, were useful in reducing the effects of salinity stress. The lowest level of malondialdehyde was observed at 0.5 mM salicylic acid without NaCl. However, no significant difference was found between salicylic acid levels.
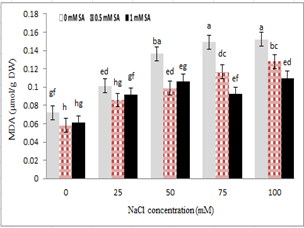
Figure 7- Effect of sodium chloride and salicylic acid on malondialdehyde content in rice
Excessive accumulation of cations and anions in the soil solution would cause salinity stress. Cell membrane has been one of the primary objectives of many environmental stresses, and maintaining the stability of the membrane under stresses such as salinity and drought has been a sign of tolerance to stress and drought [73]. Salinity stress reduced the cellular membrane clotting and release of electrolytes and intracellular materials, and increased the lipid peroxidation of cell membrane. In effect to the lipid peroxidation of membrane, compounds such as malondialdehyde, propanal, butanal, dimethyl acetal, etc. were released. These materials have been used as an indicator for measuring the amount of peroxidation of lipids [74].
Another mechanism of resistance to environmental stresses, such as salinity, was dehydration stress associated with lipid and non-saturated fatty acids, which during stress sustained the membrane [75]. The use of salicylic acid exogenous slightly increased MDA compared to the control (in the absence of salt). The biochemical changes that occurred under salinity stress produced various active oxygen species. Types of reactive oxygen were very toxic to the cells and made in the absence of a strong protective mechanism which damaged the normal metabolism of lipids, proteins and nucleic acid [76], which resulted in peroxidation of membrane lipids [77]. In this study, malondialdehyde content increased under salinity stress. Salinity stress reduced the integrity of the cell membrane, and released electrolytes and intracellular materials. When salicylic acid alone was used, malondialdehyde content was not changed, but when salinity and salicylic acid were used together, malondialdehyde content reduced, which could be due to the ability of salicylic acid to prevent the production of free radicals, because these free radicals caused peroxidation of lipids and changed the synthesis of cell membrane micro-molecule and cytoplasm. Similar results were obtained in plants such as cassia [78], pearl [79], wheat [80] and maize [81]. Salinity stress increased the lipid peroxidation of beet leaves (Beta vulgaris), Bor et al. (2003) [82], barley [41], lentil [83], spinach [59] which was consistent with the study results (Fig.7).
Increased levels of malondialdehyde due to salinity stress and its reduction due to the application of salicylic acid have been observed in lemna [84], and maize [59] and the risk of oxidative stress was reduced. The use of salicylic acid at a concentration of 0.5 mM showed a significant reduction in malondialdehyde concentration. Salicylic acid protected maize from oxidative stress somehow with reducing the peroxidation of lipids through its effect on enzymatic and non-enzymatic defense mechanisms [81].
The effect of sodium chloride and salicylic acid on the activities of antioxidant enzymes
The activity of antioxidant enzymes was heavily influenced by salinity stress. The activity of catalase was significantly increased at the highest concentration of salt and without salicylic acid treatment compared to the control treatment, and salicylic acid at a concentration of 0.5 mM reduced the activity of catalase in treatments of 100, 50 and 25 mM NaCl but in 75 mM treatment, no significant difference was observed. Increasing salicylic acid concentration to 1 mM also increased the activity of catalase.
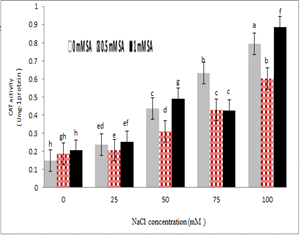
Figure 8- Interaction effect of sodium chloride and salicylic acid on activity of catalase in rice shoot
The results indicated that ascorbate peroxidase had the highest activity in 100 mM salinity stress and without the use of salicylic acid. Ascorbate peroxidase activity also increased with increasing salinity levels. A reduction in ascorbate peroxidase activity was observed due to the application of salicylic acid at a concentration of 0.5 mM, and under salinity stress conditions. Also, treatments with 1 mM salicylic acid and 100 mM NaCl reduced the activity of this enzyme with no effect other levels of stress. The 1 mM of salicylic acid stimulated the activity of this enzyme.
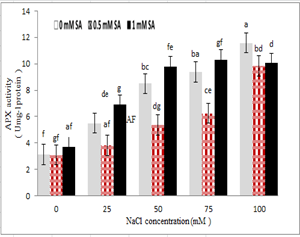
Figure 9 – Interaction effect of sodium chloride and salicylic acid on the activity of ascorbate peroxidase in rice shoot
Biological and biological stresses, such as salinity stress caused stress production. It would depend on the signs of oxidative stress on the plants, the change in the activity of antioxidant enzymes, and the sensitivity to oxidative stress depended on the pathogens and the magnitude of the antioxidant compounds in the plants [85]. Plant cells had very strong internal oxidation and rehabilitation [86]. There were two lines of defense against a wide accumulation of ROS that included both enzymatic and enzymatic activities. Non - enzymatic oxidant system including b - carotene, lycopene (antioxidants fat-soluble), dissolved in water could be such as ascorbic acid, antioxidant glutathione, carotenoids, flavonoids, phenolic compounds of proline. The enzymatic antioxidant system contained Superoxide dismutase (SOD), Catalase (CAT), Ascorbat peroxidase (APX ) and other enzymes [87].
Study of the antioxidant activity of Neda cultivar showed that salinity significantly increased the activity of antioxidant enzymes catalase and ascorbate peroxidase. The antioxidant enzymes such as catalase and Ascorbat peroxidase are enzymes that play an important role in response to abiotic stresses, including salinity. Increasing levels of antioxidant enzymes was a secondary defense mechanism against oxidative stress [88, 89]. Similar to the study results, antioxidant enzymes in maize [90], barely [91], and sesame [92] increased under salinity stress conditions. Studies by Sakhabutdinova et al. (2004) and Dixit et al. (2001) have shown that salicylic acid activated many protective enzymes such as catalase and ascorbate peroxidase, as well [93, 94], it could modify ROS production in plants, thus with reducing the activity of these enzymes, this radical increased in the plant [95]. These results indicated that the activity of the antioxidant system enzymes was directly and indirectly regulated by salicylic acid [96]. In this experiment, the use of salicylic acid depending on the concentration used also increased the antioxidant activity of rice compared to the control (Figures 8 and 9). Salicylic acid increased antioxidant enzymes in tomatoes and beans [97], which confirmed the study results. There have been various reports on the role of salicylic acid in reducing the effects of stress. Salicylic acid reduced the effects of salinity stress [41] with affecting antioxidant enzymes such as catalase [98], and peroxidases [41]. Salicylic acid derivatives also reduced the inhibitory effects of salinity and drought stresses in wheat [99], which was consistent with the study results.
Salicylic acid at low concentrations due to stimulation of the plant’s antioxidant system played a role in increasing tolerance to salinity, but at higher concentrations as a non-enzyme antioxidant it interacted with antioxidant enzymes [100]. The use of salicylic acid affected the speed of the formation of various ROS under stress conditions, and changed the activity of antioxidant enzymes such as catalase, superoxide dismutase, ascorbate peroxidase and peroxidases, thereby increasing the tolerance of plants to abiotic stresses and preventing accumulation of Na⁺ and Cl⁻ ions [101]. Although radical hydrogen peroxide was toxic at high concentrations, and was degraded by catalase, ascorbate peroxidase and ascorbate glutathione antioxidant cycle, but at low concentrations, it could act as a signal to message transmission processes and activate resistant genes in the plant [102]. It seemed that salicylic acid itself was an antioxidant agent that the addition of salicylic acid reduced the probable stress and, as a result, reduced the antioxidant enzymes of catalase and ascorbate peroxidase [103].
CONCLUSION
As a conclusion, it can be concluded that salinity stress has led to a decrease in rice plant growth.
Given that salinity stressed the balance of active oxygen species, it would also disrupt the system of eliminating them, and would kill the planned death of the cell. Therefore, increasing the resistance of plants to salinity has been considered as a solution to this problem. Salicylic acid, by activating the antioxidant defense system, increased the resistance of plants to oxidative stress due to salt stress and thus rice resistance. Regarding the physiological and biochemical traits, no significant difference was found between the effects of different concentrations of salicylic acid. While its positive effect could not be ignored in improving the damage caused by salt stress. Determining the proper concentration of salicylic acid for treatment and improving the quality of plants and resistance to environmental stress such as salinity stress has been essential. The present study demonstrated that the use of salicylic acid could have a positive effect on the activity of antioxidant enzymes. Therefore, it could be said that salicylic acid treatment could be one of the suitable solutions to reduce the harmful effects of salinity stress in rice plant.
REFERENCES

