



|
The Effect of Cold in Quality of AS 71 Variety Maize Seeds in Different Moistures
Koorosh Rahbari1, Mehdi Madandoust2*, Farhad Mohajeri3, Mohammad Rahim Ovji4 |
|
1Department of Agriculture, Faculty of Agriculture, Fasa Branch, Islamic Azad University, Fasa, Iran. 2Faculty member of Plant Physiology, Fasa Branch, Islamic Azad University, Fasa, Iran. 3Faculty member of Agriculture, Fasa Branch, Islamic Azad University, Fasa, Iran. 4Faculty member of Soil Science, Fasa Branch, Islamic Azad University, Fasa, Iran. |
ABSTRACT
Seed quality is affected by many factors, including maturity time and environmental conditions. As a result, determining an appropriate maturity time by using different tests is very important. In this study, we investigated the effect of four maturity times in terms of grain moisture content (30, 35, 40 and 45%) on Zea mays L. seed vigor using the cold. The results showed that the difference in grain yields with different moisture contents at harvest time was mainly due to the diversity of seed moisture contents. Highest percentage and rate of germination were obtained from the treatment with 30% moisture content at maturity time which showed no statistically significant difference with 35% moisture content. At maturity time, Different treatments in terms of grain moisture content caused changes in uniformity and germination index. On the one hand, treatment with 45% moisture content at maturity time delayed germination. Based on Guasnr’s (2007) equation, the nonlinear model of germination response curve was also fitting to the percentage of moisture contents at maturity time for test. In general, due to the reduction in the slope of germination response curve at high moisture content at maturity time for test, 30 and 35% moisture content at maturity time were found to be appropriate to produce high quality Zea mays L. seeds.
Key Words: Maturity time, Kernel, Cold, Seed, Maize.
INTRODUCTION
Zea mays L. is one of the most important crops grown in many countries around the world. This plant, with a global annual production of 826 million tons, can be used as one of the best crops. It is also one of the most productive cereals so that after wheat and rice, it is totally the third largest produced cereal crop in the world [1]. Moreover, there is a prediction stating that considering the world's growing population, food production will be limited to 9 billion people by 2050. Thus, Zea mays L. has good potential to meet the food demand of developing countries [2].
Since seed germination is one of the most important stages in crop growth which needs to be successfully passed, the use of high quality seeds is necessary to achieve proper yield. [3] Furthermore, seed is the most important input of agriculture sector that provides the ground for plant growth, its survival, and development. Therefore, recognizing factors affecting seed production and its quality at production and maturity stages is very important. Seed quality, indeed, indicates the combined effect of environmental conditions in which the seed has been produced, matures, and stored [4]. In most plants, seeds are able to germinate before physiological maturity so that some seeds acquire the ability to germinate only a few days after fertilization and before the usual maturity time [5]. On the other hand, early harvest of seeds can reduce their vigor. However, with increasing seeds size and maturity, their vigor gradually increases. Also, seed maturity has a significant effect on seed quality, its storage and vigor [6]. Drying during seed maturity is an integral part of seed germination so that the reduction in seed moisture content after physiological maturity stage (i.e., maturity for harvest) increases its vigor. Therefore, increasing germination percentage and seed quality is achieved with appropriate moisture content in the seed [7, 8]. On the other hand, delay in seed harvest causes a significant reduction in seed quality due to seed aging, due to undesired environmental factors, seed break and damage to the embryo during harvest. Therefore, determining an appropriate harvest time could successfully effect on plant seed quality [9]. The researchers have also reprtyed that the environmental conditions could contribute to seed harvest time through affecting the seed aging [10]. To study the environmental effects, especially its interactions with seed moisture content, makes it possible to produce seeds with the desired quantity and quality [11].
Given that seeds with high germination ability do not necessarily well-germinate and are likely to produce fewer seedlings in the field, determining seed vigor using different seed tests is highly important [12]. The simplest evaluations to determine seed quality could be performed using a standard germination test. However, this test under environmental stress in the field does not lead to an acceptable estimate. Therefore, different tests along with standard germination test can provide a more complete estimate of quality [13]. Seed vigor can be tested in different ways among which only a limited number of ways are acceptable by seed experts and seed testing institutes [14, 15]. These test low temperature germination [16]. As a result, seed quality test is highly important to check the seeds for their germination ability, vigor, and health (three most important criteria for evaluating seed quality) [11]. According to the International Seed Testing Association (ISTA), seed vigor is a set of all those properties of a seed that determine the potential level of seed activity and efficiency during germination [17]. Also, the percentage of final seed germination is defined as the percentage of normal seedlings obtained at the end of standard germination test period under desired conditions for germination [18].
According to the related literature, the important point in production of plant seed is to increase seed vigor by determining appropriate seed moisture content at maturity time. The researchers' reports have shown that timely harvest has successful effects on plant seed quality. However, there is a lack of scientific knowledge about the effect of environmental factors on plant seed vigor. Hence, better management of harvest time under different test conditions was part of this work’s innovation, which can be useful for Zea mays L. seed producers. Therefore, in this study, we evaluated different seed moisture levels at harvest time based on Zea mays L. seed vigor under test conditions.
MATERIALS AND METHODS
An experiment was performed with a randomized complete block design in three replications during growing season of 2018-2019 in Kermanshah, Kermanshah Province, Iran located at longitude of 47 degrees and 4 minutes east, latitude of 34 degrees and 18 minutes north, and altitude of 1420 m above sea level. Also, in this study, the treatments included four harvest times in terms of grain moisture content (30, 35, 40 and 45%). Besides, the effect of these treatments on Zea mays L. seed vigor was investigated using the test, cold test.
Before performing the experiment, soil samples were first taken from depths of 0 to 30 cm and physical and chemical properties of soil were determined (Table 1). Also, the level of precipitation and the average monthly temperature during the test at the synoptic meteorological station are shown in Fig. 1.
In both years (2018 and 2019), the sowing date of seeds was April 30th and harvested date was in October. In this study, cultivar AS71 was used. Sowing seeds were put on planting rows of 5 m long, with row spacing of 75 cm and density of 9 plants per square meter. The required Nitrogen for the plants was provided from the source of urea fertilizer (500 kg / ha) in three equal parts before flowering. In order to supply phosphorus, 150 kg of diamonium phosphate per hectare was used before planting. Regarding potash, before planting, 100 kg / ha of potassium sulfate was uniformly applied below the planting lines as strips. During the growing season of Zea mays L., weeding in the plots was done by hand. The plots were then irrigated in the form of ridge-furrow. The level of used water was measured by a meter installed in the field, which was 9000 cubic meters per hectare. Each experimental plot consisted of 4 planting rows so that in each plot, there were two marginal rows of paternal base and two middle rows of maternal base. The maternal base ears were covered with envelopes immediately after emergence to protect external pollen. Since acceptance of male gametophyte of the mentioned ears, coincided with the beginning of pollination of the male flowers in the paternal rows, envelopes were placed on the male flowers overnight to collect the pollen. The envelope containing the pollen was then placed on the ear in the middle rows for fertilization. Next, pollen collection was repeated for three nights to ensure complete grain closure in rows (to prevent bareness). Finally, in order to conserve the ears, the envelope remained until the end of pollination period. The seeds were harvested when the ears reached the desired moisture content. Moreover, in order to measure seed moisture content, moisture content was frequently determined by a digital hygrometer [19]. The seeds were then separated from the ears to determine both their yields and germination characteristics in different seed tests.
Table 1. The soil physical and chemical analysis.
|
Year |
Texture |
EC |
O.C |
N |
pH |
P |
K |
Zn |
Mn |
Fe |
Cu |
|
(dSm1) |
(%) |
- |
(mg Kg -1) |
||||||||
|
2018 |
Sandy loam |
0.9 |
1.01 |
0.101 |
6.7 |
12 |
540 |
0.30 |
0.8 |
5.7 |
0.03 |
|
2019 |
Sandy loam |
0.7 |
1.03 |
0.106 |
6.5 |
13 |
500 |
0.32 |
1.1 |
5.6 |
0.04 |
Note: (O.C): Organic carbon.
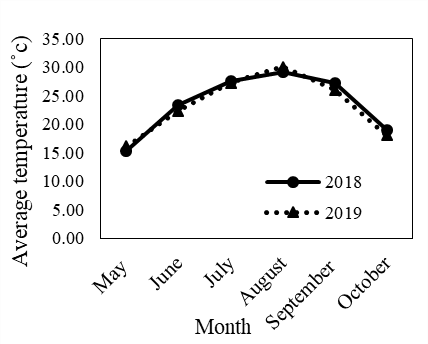
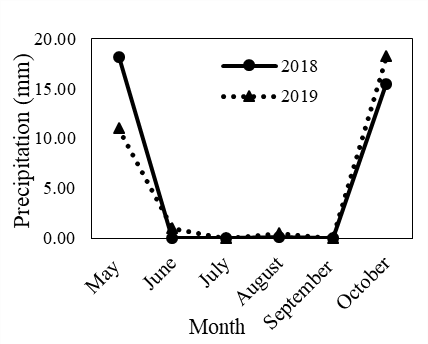
Fig. 1: The climate conditions for the seasonal patterns from May to October 2018 and 2019.
(National Meteorological organization, Iran).
After physiological maturity stageand removing two rows of the margins in each plot, an area equivalent to three square meters was harvested to determine grain yield. Finally, grain yield was reported with initial moisture content at harvest time and 14% moisture content during 2018-2019.
The Germine software was used to calculate the maximum germination, germination rate, and germination uniformity [20]. Using this software, the time to onset of 50% germination (H50) was calculated through linear interpolation method in cumulative germination curve.
Germination rate (R50) was also evaluated inversely to the time of reaching 50% of the maximum germination percentage by Eq. (1).
Germination rate R50 = 1 / H50 (1)
In this study, germination uniformity (GU) is refered to the time during which germination incresaes from 10% maximum (H10) to 90% maximum (H90), which was calculated by Eq. (2) [20].
Germination uniformity GU = D90 - D10 (2)
Germination index (GI), or in other words, the mean germination time was further determined by Eq. (3).
GI= (10n1+9n2+...+1n6/ (10N (3)
Also, germination properties H5 (time to 5% germination), H10 (time to 10% germination), H50 (time to 50% germination time), H90 (time to 90% germination), and H95 (time to 95% germination) were determined according to Guasnr’s (2007) model. In addition, germination description process was performed based on this model.
In order to perform cold test, the seeds were incubated for 5 days at 5 ° C and another 7 days at 25 ° C. At the end of the test, germination indices and seed vigor were evaluated according to standard germination model [15].
Analysis of data variance for cold test was performed using the SAS statistical software version 9.1. means of different tratment were compared using the Duncan test at the probability level of 5%.
RESULTS
Higher moisture content at harvest time caused a significant difference in maize grain yield (Table 2) So that the highest grain yield was observed in the early harvest treatment with 45% moisture during 2018-2019 with an average of 4.66 and 4.52 tons per hectare, respectively. Also, the lowest grain yield was obtained from 30% moisture treatment, which was not significantly different from 35% moisture. On the other hand, Moisture content reduction to 14% caused no significant difference in grain yield (Table 2). However, grain yield in different moisture treatments at harvest time with 14% moisture content showed no statistically significant difference. Therefore, it can be stated that differentgrain yields with different moisture contents at harvest time was mainly due to varied seed moisture contents.
Table 2. Comparison of grain yield with moisture content time of harvest and grain yield with moisture content of 14% (2018 and 2019) in corn
|
Year |
Seed Moisture Content (%) |
Grain Yield with Moisture Content Time of Harvest (t h-1) |
Grain Yield with Moisture Content of 14% (t h-1) |
|
2018 |
30 |
4.34 b |
2.97 a |
|
35 |
4.43 b |
2.84 a |
|
|
40 |
4.58 a |
2.81 a |
|
|
45 |
4.66 a |
2.79 a |
|
|
2019 |
30 |
4.21 c |
3.22 a |
|
35 |
4.27 c |
3.09 a |
|
|
40 |
4.40 b |
2.90 a |
|
|
45 |
4.52 a |
2.66 a |
In 2018, the highest germination of Zea mays L. was obtained from 30% moisture content treatment at maturity time with a mean of 96%, which was not significantly different from 35% moisture content at maturity time (Table 4). However, in 2019, germination in different moisture content treatments at maturity time showed no statistically significant difference. In 2018, germination rate was higher in 30% moisture content treatment at maturity time. Also, in 2019, germination rate with 30 and 35% moisture content treatments at maturity time showed no statistically significant difference (Table 4). There also exisred no statistically significant difference in germination uniformity during 2018-2019. Hence, different moisture content treatments at maturity time had a significant effect on germination index during 2018-2019 (Table 4).
Additionally, in 2018, the shortest time until the beginning of Zea mays L. germination at different times was observed in 30% moisture content treatment at maturity time. However, 40% moisture content treatment at maturity time delayed germination of Zea mays L. seeds in the cold test (Table 5). Also, the time until the onset of Zea mays L. germination in 30 and 35% moisture content treatments at maturity time showed no significant difference.
Similar to standard germination test, the process of cumulative germination in response to different moisture content treatments at maturity time showed different patterns (Fig. 2). However, following Guasnr’s (2007) equation, the nonlinear model of germination response curve displayed relatively similar trends for different moisture content treatments at maturity time for the cold test. Furthermore, according to the reported results in Tables 3 and 4, 30% moisture content treatment at maturity time showed higher germination percentage and rate.
Table 3. Comparison of germination duration (hours) corn harvested in different humidity (2018 and 2019) under standard germination test conditions (based on model GUASNR, 2004).
|
H95 |
H90 |
H50 |
H10 |
H05 |
Seed Moisture Content (%) |
|||||
|
2019 |
2018 |
2019 |
2018 |
2019 |
2018 |
2019 |
2018 |
2019 |
2018 |
|
|
202.0 a |
193.6 ab |
196.0 a |
183.3 a |
143.6 a |
142.3 a |
81.7 a |
87.0 b |
63.5 a |
72.0 b |
30 |
|
176.4 a |
194.7 ab |
169.4 a |
188.6 a |
130.7 a |
147.7 a |
81.4 a |
96.0 b |
70.4 a |
74.5 b |
35 |
|
186.9 a |
188.0 b |
181.1 a |
183.5 a |
142.3 a |
151.1 a |
99.8 a |
105.5 b |
82.3 a |
83.6 b |
40 |
|
183.5 a |
210.5 a |
174.7 a |
202.4 a |
131.9 a |
164.1 a |
96.5 a |
131.6 a |
84.0 a |
110.3 a |
45 |
Similar letters in each column indicate non-significant difference according to Duncan test at the 5% level.
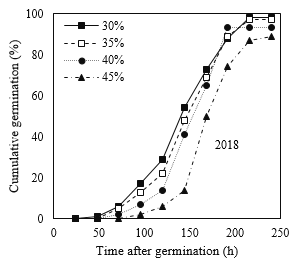
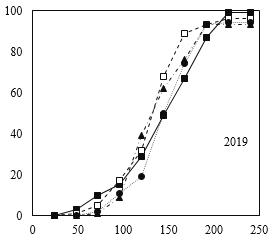
Fig 2. Percentage of germination of corn seeds in different moisture harvests (2018 and 2019) during different times after germination under standard germination test conditions.
Table 4. Comparison of germination characteristics of corn seeds in different moisture harvests (2018 and 2019) under cold test conditions.
|
Germination Index |
Germination Uniformity (hour) |
Germination Rate (per hour) |
Germination Max (%) |
Seed Moisture Content (%) |
||||
|
2019 |
2018 |
2019 |
2018 |
2019 |
2018 |
2019 |
2018 |
|
|
5.1 b |
5.3 c |
93.6 a |
87.4 a |
0.0086 a |
0.0086 a |
96 a |
96 a |
30 |
|
5.4 b |
5.8 b |
102.1 a |
91.1 a |
0.0083 a |
0.0076 b |
95 a |
93 ab |
35 |
|
6.1 a |
6.2 b |
98.2 a |
89.5 a |
0.0066 b |
0.0073 b |
91 a |
90 bc |
40 |
|
6.1 a |
6.8 a |
79.8 a |
72.6 a |
0.0073 b |
0.0063 c |
91 a |
88 c |
45 |
Similar letters in each column indicate non-significant difference according to Duncan test at the 5% level.
Table 5. Comparison of germination duration (hours) corn harvested in different humidity (2018 and 2019) under cold test conditions (based on model GUASNR, 2004).
|
H95 |
H90 |
H50 |
H10 |
H05 |
Seed Moisture Content (%) |
|||||
|
2019 |
2018 |
2019 |
2018 |
2019 |
2018 |
2019 |
2018 |
2019 |
2018 |
|
|
169.5 a |
171.0 b |
158.2 a |
160.1 b |
114.7 b |
116.2 c |
64.6 b |
72.7 c |
52.0 b |
60.7 c |
30 |
|
178.9 a |
183.8 ab |
169.2 a |
174.7 ab |
120.3 b |
131.3 b |
67.1 b |
83.5 bc |
59.2 b |
70.4 bc |
35 |
|
191.3 a |
188.0 ab |
180.4 a |
181.0 a |
142.3 a |
137.7 b |
82.1 ab |
91.4 c |
71.9 ab |
81.9 b |
40 |
|
184.9 a |
199.9 a |
173.4 a |
187.3 a |
135.6 a |
156.7 a |
93.6 a |
114.6 a |
83.7 a |
106.7 a |
45 |
Similar letters in each column indicate non-significant difference according to Duncan test at the 5% level.
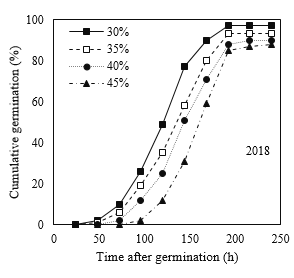
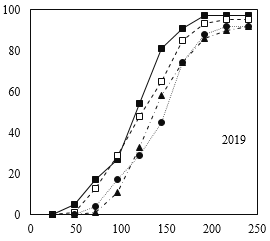
Fig 3. Percentage of germination of corn seeds in different moisture harvests (2018 and 2019) during different times after germination under cold test conditions.
DISCUSSION
According to the observed results, harvesting after physiological maturity stage did not affect grain yield So that the difference in yields of different treatments at harvest time was mainly due to the difference in seed moisture. Therefore, maximum seed dry weight or weight maturation occurred after physiological maturation. It has been also reported that the highest grain yield is achieved after physiological maturity, when athe seed dry weight will not change [21]. However, different harvest times could diversly affect germination indicators [22].
Furthermore, the obtained results verified that early harvest with 40 and 45% moisture content treatments had strongly affected germination percentage and rate of Zea mays L. on the other hand, seed aging due to degradation of cell membrane structure under environmental conditions during improper seed harvest has been introduced as a reducing factor in seed vigor [23]. However, in the present work, the harvested seeds with 30% moisture cotent seemed to have the least pre-harvest aging and therefore had the highest germination. In general, germination does not occur even under the desired conditions until the seed has completed the minimum morphogenic stages required for embryonic development [24]. The larger and more mature the seed is the more the seed vigor is; therefore, increasing germination percentage along with reducing seed moisture content at maturity time can be expected [8].
The germination percentage in the cold test showed a slight change compared with the standard test, and the seed germination rate was somewhat higher. Therefore, environmental factors such as cold could be effective on the difference on various germentation indicators with different moisture content treatments at maturity time. It has been also stated that cold-pretreatment changes the indicators related to seed germination and vigor [25]. The reason for this difference is activatig the physiological mechanism of seeds under different temperature conditions [26]. Therefore, cold-pretreatment could accelerate germination process through affecting the embryo as well as the stimuli of the germination process, including gibberellic acid [27].
In this study, different maturity times were considered with their effect on germination indicators in the accelerated aging test. In this regard, we found that increase in both temperature and seed degradation could adversely affect germination-related traits of seed. This is especially important under long storage ae well as hot and moist climate conditions [28]. Reduction in seed vigor has been reported as the effect of accelerated aging test on seed quality due to the complete degradation of the cytoplasmic membrane structure of seed cells [10]. However, the accelerated aging test reduces vigor of Zea mays L. seeds, particularly due to improper harvest time. Thus, damage to embryonic cells, especially the cell wall structure is due to high temperature and moisture [29]. In general, aging reduces germination indicators by affecting the activity of hydrolyzing enzymes and other cellular systems that transfer seed stock material [30].
Improper harvest time leads to poor quality seed, which have a weak membrane structure to absorb suficient water during imbibition. Due to the damaged seed coat, material leakage from the seed coat also increases [31]. As seen, Results of the brick test clearly confirmed the reduction in germination indicators with inappropriate maturity moisture content. The reduction in the slope of germination response curve to the percentage of moisture content treatments at maturity time was evident for the brick test. This test provided mechanical stress for germinating seeds. The high correlation of the brick test results has also been reported with field results for seed germination [32, 33].
There have been many reports on reduced germination and related indicators in seeds affected by drought stress test with polyethylene glycol solution [34, 35]. The reduction in the slope of germination response curve to moisture content treatments at maturity time for drought stress test was also quite evident in this study. Therefore, seeds that could show higher germination percentage and rate under drought stress conditions of 0.5 and 1 MPa can be reported as the superior treatment, which, in this study, was 30% moisture content treatment at maturity time. Some researchers have attributed such a response to the reduction in water absorption by seeds treated with polyethylene glycol [36]. Drought stress also affects germination by influencing the movement and transfer of seed stock. Polyethylene glycol reduces hydrolysis of seed stock material by creating drought stress conditions and subsequently reducing germination percentage [34].
CONCLUSION
The study results showed that the main difference in yields of different treatments at harvest time was due to the difference in seed moisture contents. Therefore, the maximum seed dry weight or weight maturity occurs after physiological maturity. Results of this study showed that seed quality and its various aspects could be affected by environmental conditions applied by seed vigor test. For example, a reduction in the slope of germination response curve to moisture content treatments at maturity time was observed for the accelerated aging, brick, and drought stress tests. The obtained results also indicated that environmental conditions during seed maturity time may have a role in seed vigor reduction by affecting seed aging process. The quality of Zea mays L. seeds with 30% moisture content was higher than other moisture contents, while seeds treated with 35% moisture content were ranked second. On the other hand, treatments with 40 and 45% moisture content at maturity time significantly reduced germination process. As a result, 30 and 35% moisture content treatments at maturity time under the influence of different environmental conditions is recommended to produce high-quality seeds of Zea mays L.
ACKNOWLEDGEMENTS
Hereby, we would like to express our appreciation of Zea mays L. Cultivation Development Institute as well as the Islamic Azad University, Fasa Branch, Iran sincere assistance in fulfilling this study.
REFERENCES
ination. Journal of Plant Nutrition. 2019 Dec 14;42(20):2814-23.