|
The diagnostic value of serum Neopterin levels in patients with newly diagnosed pulmonary/extra-pulmonary Tuberculosis
Shokrollah Salmanzadeh 1*, Seyed Mohammad Alavi 2, Elham Senisel Bachari 3 |
|
1 Assistant Professor of Infectious and Tropical Diseases, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. 2 Full Professor of Infectious and Tropical Diseases, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. 3 Specialist in Infectious and Tropical Diseases, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. |
ABSTRACT
Background: Tuberculosis (TB) is still a primary public health concern worldwide, the most common of which is pulmonary involvement. The official diagnosis of tuberculosis involves the culture of a tissue sample, which in turn takes time. Neopterin, as one of the indicators of cellular immune activity, is secreted by macrophages and T lymphocytes in different inflammatory phenomena due to the stimulation of interferon-gamma. Therefore, it may be possible to measure the serum level of Neopterin in the diagnosis and follow-up of the response to pulmonary and extra-pulmonary tuberculosis treatment. Objective: This study aimed to evaluate the serum level of Neopterin in the diagnosis of newly diagnosed pulmonary/extra-pulmonary tuberculosis. Method: Six groups of 20 individuals, including pulmonary TB, extra-pulmonary TB, healthy, proven rheumatologic diseases, patients with pulmonary malignancies, and finally, those with lung infections, entered the study. A serum sample was taken to assess the level of Neopterin. The samples were transferred to the laboratory in less than 30 minutes. Results: The level of Neopterin is significantly higher in patients with pulmonary TB into patients with extra-pulmonary TB, healthy and lung infections, and then in extra-pulmonary TB was significantly higher than in healthy individuals, patients with lung cancer and lung infection. Conclusion: According to the significant relation between serum Neopterin level in patients with pulmonary and extra-pulmonary TB compared to healthy people without any underlying disease, it may be possible to initiate specific pulmonary tuberculosis treatment in a shorter period. However, it is necessary to follow the tissue culture sample.
Key Words: Neopterin, Tuberculosis, pulmonary TB, extra-pulmonary TB.
INTRODUCTION
Tuberculosis is one of the ten leading causes of death worldwide and the leading cause of death from an infectious agent. Millions of people are infected by TB each year. In 2018, 1.5 million people died of tuberculosis, 300,000 of whom had pulmonary TB. About 10 million people became infected with tuberculosis this year, including 5.7 million men, 3.2 million women, and 1.1 million children. There have been cases in all countries and age groups, but tuberculosis is treatable and preventable [1]. In addition to pulmonary tuberculosis, extra-pulmonary types including lymph node TB, pericarditis TB, myocardial TB, laryngitis TB, meningitis TB, the involvement of the digestive system, and the involvement of the genital administration system have also been reported [2, 3]. One of the goals of Sustained Development Goals (SDGs) for countries, developed by the United Nations in 2015, is to end the TB epidemic by 2030. The WHO End TB approach adopted by the World Health Organization in 2015, is a 90% reduction in TB deaths and an 80% reduction in the incidence of the disease by 2030 [4, 5]. Still, the standard diagnostic method in TB patients is direct observation of bacilli in sputum smears, and this method is also used to monitor and follow up the response to treatment. Diagnosis of extra-pulmonary tuberculosis is more complicated than pulmonary tuberculosis and requires the use of invasive diagnostic methods such as biopsy and tissue culture. Therefore, using methods that can help faster diagnosis, without using invasive techniques, will be very useful [6].
Neopterin (NPT) (6-D-erythro-trihydroxypropyl pteridin) is one of the indicators of the activity of the cellular immune system in the body and its level of serum increases in infections that stimulate the immune system [7]. Neoprene was first isolated from bee larvae and royal jelly in 1963, and then in 1967 separated from humans [8]. It produced from the conversion of guanosine triphosphate to neopterin is involved in T lymphocytes stimulated by interferon-gamma and to a lesser extent by interferon-alpha and beta, as well as in macrophages, dendritic and endothelial cells, and to a lesser extent in fibroblasts, smooth vascular wall muscles, and renal epithelial cell [9, 10]. In the case of non-mycobacterial bacterial infections, studies have shown that the urinary excretion of neoprene is lower than that of viral infections [11, 12]. However, in pulmonary infections caused by Mycobacterium tuberculosis, the amount of urinary neoprene is higher than that of bacterial pneumonia and cancer [13]. According to the above mentioned, the availability of a non-invasive method for the diagnosis of extra-pulmonary tuberculosis is essential. This study aimed to investigate the diagnostic value of serum neoprene as a method for diagnosing extra-pulmonary tuberculosis to diagnose the disease by spending less time and money without using invasive techniques.
METHOD
In the current study, serum levels of neoprene were measured in patients with extrapulmonary TB and compared with serum levels in extra-pulmonary TB, healthy, proven rheumatologic diseases, patients with pulmonary malignancies, and finally those with lung infections. Plasma neopterin levels were determined using high-efficiency fluid chromatography with fluorimetry detection [14]. To determine the sample size, we used a comparison formula of two averages, so the sample size included six groups of 20 individuals and total control of 120 individuals. Inclusion criteria were patients with pulmonary tuberculosis based on the national protocol with at least two samples of positive sputum smear, or a positive smear sample and clinical symptoms corresponding to tuberculosis, or a positive smear and radiographic evidence of pulmonary tuberculosis, or a positive smear sample with a positive culture of Mycobacterium tuberculosis. It is noteworthy that the condition for entering the recent study is the time range immediately after the diagnosis of tuberculosis and before starting the treatment. Patients with newly diagnosed extracellular tuberculosis entered the study using one of the following methods and before receiving any medication that affected tuberculosis; Pathological evidence for tuberculosis, such as observations of cohesive granuloma, a positive PCR test on the patient's tissue sample, and direct view of the bacillus tuberculosis on the tissue sample before starting anti-tuberculosis drug therapy. Exclusion criteria were patients who had started anti-tuberculosis treatment before sampling, with extrapulmonary tuberculosis as well as tuberculosis, cancer, rheumatoid diseases, bacterial pneumonia, hepatitis or HIV, patients with defective immune system any reason, and consumers of drugs that weaken the immune system. In the present study, participants were divided into three age groups of less than 35 years, 35 to 50 years, and over 50 years.
Statistic
Data are expressed as the mean ± standard deviation. Significance was established with the SPSS 21.0 statistical (IBM Corp., Armonk, NY, USA). Turkey conducted comparisons between two groups. P<0.05 was considered to indicate a statistically significant difference.
RESULTS
The overall results illustrated some of the main characteristics of the study participants. It can be seen from the data in Table 1 that six study groups consisted of both men and women each included 20 people. The number of participants in each group of the male and female, mean and standard deviation of age, and the mean and standard deviation of the serum level of neopterin are listed in Table 1. As can be seen, the population of men and women in this study is generally equal, and the average age of the participants in different groups did not differ significantly.
Table 1: Characteristics of the study participants
|
Groups/value |
Pulmonary TB |
Extrapulmonary TB |
Rheumatic Diseases |
Pulmonary Malignancies |
Pulmonary Infections |
Healthy |
|
|
Number of Participants |
20 |
20 |
20 |
20 |
20 |
20 |
|
|
Gender |
Male |
12 |
13 |
11 |
10 |
10 |
6 |
|
Female |
8 |
7 |
9 |
10 |
10 |
14 |
|
|
Age Mean±SD |
60.9±18.01 |
39.65±18.82 |
48.05±14.69 |
50.30±14.74 |
63.05±21.66 |
42.50±12.44 |
|
|
Less than 35 years % |
5% |
50% |
20% |
10% |
15% |
35% |
|
|
35 – 50 years % |
25% |
10% |
35% |
45% |
20% |
35% |
|
|
Over 50 years % |
70% |
40% |
45% |
45% |
65% |
30% |
|
|
Neopterin nmol/L mean±SD |
35.55±23.21 |
15.75±7.29 |
16.6±16.77 |
27.24±23.36 |
31.47±26.77 |
6.7±1.75 |
|
The gender, age, and serum level of neopterin in six study groups. Data are expressed as mean±SD, P<0.05 was considered to indicate a statistically significant difference.
As can be seen in Table 2, serum neopterin variation in different disease groups is diverse, but none of them has a reduced level. All cases with pulmonary tuberculosis had elevated levels of neopterin and all healthy people had normal values of neopterin. The number of rheumatoid arthritis people with normal and increased levels of neopterin was equal, and in the other three groups, most participants exhibited increased levels of neopterin (Table 2).
Table 2. Neopterin variation in different groups
|
Groups |
Variation |
Frequency |
Percent |
|
Pulmonary TB |
increased |
20 |
100.0 |
|
Extrapulmonary TB |
normal |
2 |
10.0 |
|
increased |
18 |
90.0 |
|
|
Rheumatic Diseases |
normal |
10 |
50.0 |
|
increased |
10 |
50.0 |
|
|
Pulmonary Malignancies |
normal |
3 |
10.0 |
|
increased |
17 |
85.0 |
|
|
Pulmonary Infections |
normal |
7 |
35.0 |
|
increased |
13 |
65.0 |
|
|
Healthy |
normal |
20 |
100.0 |
Figure 1 compares the results obtained from the preliminary analysis of neopterin in groups. It is apparent from this figure that there was a significant difference between the Neopterin level among pulmonary tuberculosis patients against healthy individuals and extrapulmonary TB. There is a significant difference between the healthy, pulmonary TB, pulmonary malignancies, and pulmonary infections groups. Further analysis showed that neopterin level in the extrapulmonary TB group is significantly lower than pulmonary TB, pulmonary malignancies, and pulmonary infections groups. No significant differences were found between rheumatoid patients and others (Figure 1).
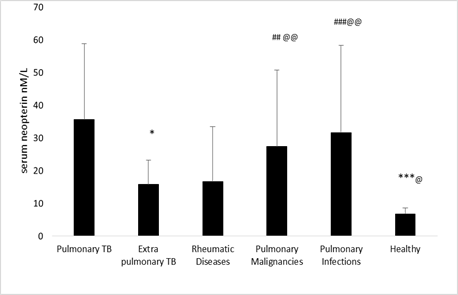
Figure 1. The neopterin level in groups. Data are expressed as mean±SD. P<0.05 was considered to indicate a statistically significant difference.* p<0.05, *** p<0.001 the pulmonary TB vs other groups. ## p<0.01, ### p<0.001 healthy vs. other group. @ p<0.05, @@ p<0.01 the extrapulmonary TB vs other groups.
DISCUSSION
Tuberculosis is a common infectious disease with tenth-degree global morbidity and, in many cases, it leads to death. It is challenging to diagnose active tuberculosis solely based on signs and symptoms. It is also difficult to diagnose it in patients with immunodeficiency [15, 16].
This study aimed to compare the serum levels of neopterin, as an inflammatory factor, in several groups of patients with common clinical symptoms. The highest amount of neopterin in the serum was in pulmonary TB, pulmonary infections, Pulmonary Malignancies, Rheumatic Diseases, Extrapulmonary TB, and healthy groups, respectively. High levels of neopterin in TB have been well discussed in the literature. Various studies have shown that in response to anti-tuberculosis treatment, the level of this factor becomes comparable to healthy people or non-tuberculosis diseases, such as pneumonia and malignancy [17].
Contrary to expectations, this study did not find a significant difference between serum neopterin levels among patients with rheumatic diseases and other groups, and also no significant relation was reported between serum levels of neopterin by age and sex of individuals. However, according to some studies, tuberculosis and serum neopterin can be affected by age and gender [18].
In the current study, the mean serum neopterin levels were 15.75 nmol/L and 6.7 nmol/L in extrapulmonary TB patients and healthy controls, respectively. Our findings were consistent with the results of a similar study in which serum neopterin levels were significantly increased in the group with pulmonary tuberculosis and extrapulmonary TB, compared with the healthy control group [19]. Also, serum levels of neopterin in patients with pulmonary tuberculosis in this study were similar to those of the study by Goyal et al. in 2017 [20].
To answer the question of whether a neopterin is a useful marker for the early detection of pulmonary extracellular tuberculosis, we tend to the significant difference of this factor in several groups. The current study revealed that serum levels of neopterin in patients with extrapulmonary tuberculosis were also significantly different than those in the group of pulmonary malignancies, pulmonary infections, and healthy individuals. Eisenhut, in 2016 found a significant association between serum neoprene levels in patients with extrapulmonary tuberculosis compared with viral and bacterial pneumonia as well as healthy individuals. Besides, the average serum level of neopterin in patients with extra-pulmonary tuberculosis was higher than the others [21].
In the current experiment, the amount of neopterin in pulmonary TB and pulmonary infections were similar. However, the findings of the current study do not support the previous research, in which patients diagnosed with bacterial infection with species other than mycobacteria showed significantly lower levels of urinary neopterin than patients with viral infections but did not differ statistically [22].
CONCLUSION
The main goal of the current study was to determine the rapid differential value for neopterin in pulmonary and extrapulmonary tuberculosis. These findings suggest that in general, the serum level of the neopterin is most likely to be used to diagnose pulmonary and extrapulmonary tuberculosis and to differentiate pulmonary tuberculosis from pulmonary infections and pulmonary malignancies. However, by this study alone, it is not possible to comment definitively on the differentiation of extra-pulmonary tuberculosis from another pulmonary disease, and further studies are needed. Taken together, these results suggest that other immune system markers secreted during TB disease should be evaluated.
REFERENCES

