



|
Physicochemical Evaluation and Chromatographic Studies of Plantago Lanceolata Grown in Northern Border Province, Saudi Arabia
Naira Nayeem 1*, Mohd. Imran 1, Hayet Mohamed Ben Khaled2 |
|
1 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Northern Border University, Saudi Arabia. 2 Department of Basic and Health Sciences, Faculty of Pharmacy, Northern Border University, Saudi Arabia. |
ABSTRACT
Plantago lanceolata known as Azan el-kabsh in Arabic; belongs to the family Plantaginaceae and is wildly grown in Saudi Arabia. It is reported to be used as emollient, astringent expectorant, in gastrointestinal disorder, as anti-inflammatory, anti-spasmodic, in wound healing, as antibacterial and antiviral. The study is designed with an aim to carry out the physicochemical parameters in accordance with the World Health Organization guideline /pharmacopeial standards and chromatographic analysis for identifying some compounds. The parameters evaluated include ash values, loss on drying, extractive value, moisture content, fluorescence analysis, TLC studies and HPLC analysis. The total ash was found to be 9.8%, acid insoluble ash 2.7 %, and water-soluble ash was 5.6%. The sample was dry but hygroscopic, with a loss on drying of 3.0 %. The methanolic extractive value of the drug was found to be 12.7% (%w/w) and was highest, which indicates the presence of polar components while the least extractive value was obtained in the ethyl acetate extract i.e. 1.2%. The TLC screening showed the presence of caffiec acid and ferulic acid. Caffiec acid was quantified by HPLC analysis. The amount of caffiec acid was found to be 0 .08 mg/ml and the retention time was 3.073. The study of these standardization parameters will provide referential information for the standardization and authentication of this plant.
Key Words: Plantago lanceolata, chromatographic, physicochemical, TLC.
INTRODUCTION
Plants are the oldest friends of humans and have been the subject of scholarly researches since ancient times [1, 2]. The trend of using plants both in a traditional and modern way is still popular and practiced for the prevention and treatment of certain illnesses [3, 4]. Herbs for healing various diseases and disorders have been used from time immemorial and has been followed in various cultures and civilization all over the world. Herbal medicine has become the most reliable form of alternative medicine. This has resulted in the demand for herbal products in the global market as it is believed that these herbs are safe, easily available, less expensive, and pharmacologically effective [5, 6]. Quality control of these herbal products is considered to be of great importance for the use herbs as medicine. World Health Organization (WHO) has prescribed various quality control tests to evaluate medicinal plant materials to confirm the identity, quality, purity, and detection of adulteration if present. In accordance with WHO guidelines, to ensure the reproducible quality of the herbal medicinal plant, it is important to evaluate the physicochemical and phytochemical characters to assist in identifying and finding out the purity and quality of the herb [7, 8]. Hence it becomes worthwhile to document the standardization profile of the various quality control parameters of herbal drugs.
Traditional medicine is followed in the whole of the Arab world and Saudi Arabia is no exception. Saudi Arabia’s heritage includes several homemade remedies. Flora of Saudi Arabia comprises of several medicinal plants [5, 6]. However, there are not many reports of standardization of plants used traditionally for medicinal purposes. Therefore, it is pertinent to document the of standardization paramaters so as to create a profile for the identitification of herbal drugs. Hence this project is designed to evaluate and document the physicochemical properties of the plant under consideration i.e. Plantago lanceolata (Arabic name: Azan el-kabsh) grown in Northern Border Province, Saudi Arabia. It is reported to be used as astringent, demulcent, emollient, expectorant, cough, in gastrointestinal disorder, diarrhea, diuretics, as anti-inflammatory, antibacterial, anti-spasmodic, wound healing, and antiviral [9-17].
MATERIALS AND METHODS
Plantago lanceolata was collected from Rafha, Northern Border Province, Saudi Arabia. The plant was then pulverised to obtain coarse powder and dried under shade. This was then extracted with various solvents of increasing polarity. The extracts were subjected to analysis of the chemical constituents like glycosides, alkaloids, carbohydrates, tannin, steroids, and flavonoids following standard procedures.
Physicochemical characterization of the plant:
The physicochemical properties that were evaluated were ash values, acid-insoluble ash, water-soluble ash, loss on drying, fluorescent analysis, extractive values as per standard guidelines [18-20].
Extractive values
The coarse powder of the plant material was extracted using various solvents i.e. petroleum ether, ethyl acetate, chloroform, n-hexane, methanol. The extract thus obtained was filtered, solvent was evaporated and the percentage of the extract with respect to the weight of the air-dried plant material taken was calculated.
Total ash value
The total ash value was evaluated using 1gm of accurately weighed dried powder of the leaves, followed by incinerating in a porcelain crucible at a temperature not more than 450°C until it became completely white. The crucible was allowed to be cooled, followed by weighing. This process was continued to obtain a constant weight. The total ash content was calculated as mg/g of the dried material and the amount was expressed in a percentage.
Acid-insoluble ash value
To the total ash that was obtained from the above step; 50 ml HCl was added and boiled for 5 mts. The insoluble matter obtained was transferred to an ashless filter paper and rinsed with water to obtain a neutral filtrate; this filterate was transferred back to the original crucible and ignited to get a constant weight. Acid-insoluble ash was calculated as mg/g of dried material and expressed in percentage.
Water-soluble ash value
The required ml of water was added to the crucible containing the total ash; this was then boiled, cooled, followed by filteration using an ashless filter paper. The Insoluble matter was ignited periodically to obtain a constant weight. Water-soluble ash was calculated by subtracting the residue weight in mg from the weight of total ash and expressed in percentage.
Loss on drying
1 gm sample was weighed in a tarred petri dish and dried at 105°C for around 5 hrs and weighed. This process was continued at 1 hr interval to obtain a constant weight. Result was calculated as loss of weight in percent.
Sulfated Ash Test [21].
The plant material was accurately weighed, put into a tarred crucible, and gently ignited to get a charred residue. This residue was cooled, moistened with 1 ml of H2SO4, and was further ignited at high temperature.
Fluorescence Analysis
The sample was placed in test tubes and was examined both in daylight and UV light at 254 nm after the addition of 5 ml of various solvents like distilled water, methanol, 1M HCl, 10% NaOH, 10% CH3COOH,10% FeCl3, 10 % I2 [19]. The tubes were allowed to stand for about 10 mts. The fluorescence characteristics were further examined.
TLC analysis:
Methanolic and chloroform extracts were selected for the TLC studies. Various combinations of the solvent system were tried and the one with the best resolution was selected. The ethanolic extract sample was spotted onto the TLC plate and placed in a chamber containing the solvent system and was allowed to develop. Evaluation of separated constituents was carried out by calculating Rf values [22].
HPLC analysis
HPLC analysis was carried out using Shimadzu spd10A uv-vis, Pump: Shimadzu LC-10ATVP, software used was Baseline chromatography Data System N2000. Detection: UV-Vis Abs.-Variable Wave. (UV) @ 237nm The column used was Phenomenex Gemini-NX-5 µm C18(2) 110 Å, LC Column 250 x 4.6 mm, ambient temperature was maintained, mobile phase was Methanol: Phosphate buffer pH 3.2 (60:40), flow rate was 1mL/min.
Standard preparation
10 mg of Caffeic acid was taken in a volumetric flask and the volume was made to 10ml using methanol as solvent. From this 1 ml of the solution was pipetted in 10ml of volumetric flask and the volume was made up with methanol,followed by sonification for 8 mins; this was further filtered using 0.45-micron Millipore filters.
Sample preparation
To 5 mg each of extract 5 ml of methanol was added. The sample was filtered using 0.45-micron Millipore filters. 20µl of the sample was injected into the HPLC system.
RESULTS AND DISCUSSION:
Evaluation of identity, quality, and purity is of prime importance to assure the safety of medicinal herbs. Various parameters like solvent extractive values, moisture, ash content, fluorescent, chromatographic analysis, etc can be used to establish the identity and quality of the plant drugs. Extraction using solvents of different polarities helps in identifying the solvent for quantification and isolation of the various phytoconstituents present in the plant. Usually, solvents of higher extractive values are preferred for further investigation. High alcohol soluble and water-soluble extractive values indicate the presence of polar phytoconstituents. Petroleum ether, ethyl acetate chloroform, n-hexane, and methanol (various polarities) were used as solvents for the evaluating the extractive values. The methanolic extract was 12.7 (%w/w) which was the highest, indicating polar components, while the least extractive value was obtained in the ethyl acetate i.e. 1.2%. Literature reports the extraction of different plants using different solvents by various researchers [18, 23]. The extractive values of the various solvents are as depicted in table 1.
Table 1: Extractive values and the nature of extracts
|
Sl no |
Solvent |
Color |
% yield |
|
1 |
Petroleum ether |
Green |
2.8% |
|
2 |
Ethyl acetate |
Greenish-yellow |
1.2% |
|
3 |
Chloroform |
Light brown |
9.0% |
|
4 |
n-Hexane |
Greenish brown |
6.3% |
|
5 |
Methanol |
Blackish brown |
12.7% |
The determination of ash values is significant as it helps in detecting low-grade products, presence of earthy matter, and exhausted drugs. Ash content gives an understanding about the inorganic content of the plant under investigation. Sometimes the percentage variation of ash from one sample to another may be little, but this indicates the change in quality. Ash values increase with adulteration, contamination, and substitution. Any increase in acid-insoluble ash indicates contamination with sand/soil. Water-soluble ash is an indication of the presence of exhausted material [15]. The total ash obtained was 9.8 %w/w, acid insoluble ash was found to be 2.7%. Insufficient drying may sometimes lead to enzymatic deterioration of active constituents therefore determination of moisture content is another important parameter to be assessed for plant drugs [18]. This parameter measures both water and volatile matter. Excess of water content in plant materials results in microbial growth and deterioration. Moisture content of the powdered leaf drug was 2.3 (% w/w) indicating that the drug was properly dried and stored. The amount of total ash, water-soluble ash, acid insoluble ash, sulfated ash values, and loss on drying is as in table 2.
Table 2: Ash values, loss on drying and moisture content
|
Result% |
Parameters |
Sl no |
|
9.8% |
Total ash |
1 |
|
2.7% |
Acid insoluble ash |
2 |
|
5.6% |
Water-soluble ash |
3 |
|
5.1% |
Sulphated ash |
4 |
|
3.0% |
Loss on drying |
5 |
|
2.3% |
Moisture content |
6. |
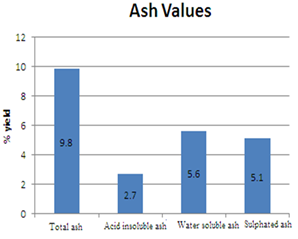
Figure 1: Ash values of the powdered material
Fluorescence study of the plant is one of the important qualitative parameter and hence it is used as an analytical tool for the identification and standardization of crude drugs. The variation of the color in the presence of visible light and UV light can serve as a guide to control the quality of herbs. Fluorescent characteristics of a drug sample indicate the presence of certain phytoconstituents that show fluorescence either in the visible range or under ultraviolet light [22]. In this study fluorescence analysis of dried powder was evaluated using different organic solvents and the results are as reported in table 3.
Table 3: Fluorescence analysis of Plantago lanceolata
|
UV |
Visible/daylight |
Powdered drug treatment |
|
Yellowish green |
Light green |
Powdered drug(PD) |
|
Reddish Brown |
Reddish yellow |
PD+1M HCl |
|
Dark brown |
Brown |
PD+10%NaOH |
|
Brown |
Light brown |
PD+1%CH3COOH |
|
Reddish-brown |
Reddish-brown |
PD+10%FeCL3 |
|
Brown |
Yellowish orange |
PD+10%I2 |
|
Orange-yellow |
Yellowish |
PD+Methanol |
TLC profile of plant extracts gives information regarding the various phytochemicals, as different constituents exhibit different Rf values when various solvent systems are used. This provides valuable information regarding the nature of polarity and also helps in the selection of the best solvent system for isolation [18]. TLC was performed on plates that are commercially available. The TLC for the methanolic extract and Chloroform extract was performed using various combinations of solvents and the solvent system having best resolution is as mentioned in table 4. The chromatogram of the methanol extract revealed the presence of eight spots. While the chloroform extract showed 03 spots. The result of derivatization of the TLC plate with ferric chloride showed brownish-yellow spots (03 spots in the methanolic extract and 01 spot in the chloroform extract) which was attributed to the presence of phenolic compounds. Table 4 depicts the extract, solvent system used, and the Rf values of the spots
Table 4: TLC analysis of the methanol and chloroform extract:
|
Rf values |
No of spots |
Detection |
Solvent system |
Extract |
|
0.05, 0.17, 0.2, 0.6, 0.82, 0.9, 0.92 |
08 |
UV 254 |
Ch: Me: water 12:7.5:3 |
Methanol |
|
0.17,0.82,0.9,0.92 |
04 |
Ferric chloride |
||
|
0.3,0.39,0.7 |
03 |
UV 254 |
n-hex: EA: AA 3.2:9.1:05 |
Chloroform |
|
0.7 |
01 |
Ferric chloride |
Chloroform: Ch, Methanol: Me, n-Hex:hexane, EA: ethyl acetate, AA: acetic acid
We had earlier reported the presence of flavonoids rutin and quercetin and phenolic acids ie gallic acid, vanillic acid, salicylic acid from this plant [24]. Several other Plantago species have been reported to contain caffeic acid, chlorogenic acid, p-cumaric acid, ferulic acid, linalool, apigenin, plantagonin, scutallarin, luteolin,aucubin, gardoside, indicain, plantagonin and many more [25]. The methanolic extract was again subjected to TLC to identify some of the other compounds from this plant. Caffiec acid, ferulic acid, and chlorogenic acid were used as biomarkers. A combination of toluene: ethyl acetate: formic acid (2: 5: 1) was used to identify these compounds followed by the use of Ferric chloride for derivatization. The results revealed the presence of caffiec and ferulic acid but chlorogenic acid was not detected by this solvent system.
HPLC analysis:
The HPLC analysis was carried using the conditions as described in the methodology. Caffeic acid was used as a marker. The results of the HPLC further authenticated the presence of Caffeic acid. The HPLC chromatogram of the methanolic extract is as seen in figure 2 while figure 3 depicts the chromatogram of standard caffiec acid. Caffeic acid showed a retention peak at 3.073 having a height of 430280 and area 7147558.5. Caffeic acid in the sample was 0 .08 mg/ml.
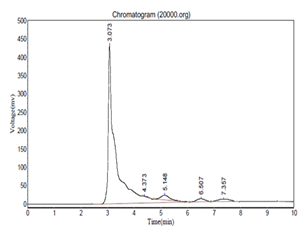
Figure 2: HPLC chromatogram for the methanolic extract
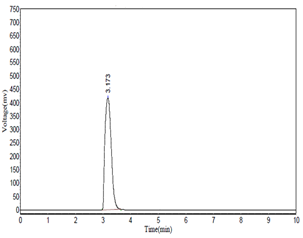
Figure 3: HPLC chromatogram the standard caffiec acid
CONCLUSION
The results of the current study establish the various physicochemical parameters and Fluorescence analysis of Plantago lanceolata. This data may help in standardizing the plant as it is an important analytical aspect for evaluating the identity, quality and purity plant drugs. The information about physiochemical properties is an indispensable aspect for authentication and development of herbal drugs from crude material. The TLC study showed the presence of caffiec acid and ferulic acid. Furthermore, the findings obtained from our study could also help in the identification, revalidation, and to evaluate the pharmacognostical properties of this plant.
ACKNOWLEDGMENT
The authors are grateful to the Deanship of Scientific Research, Northern Border University, Saudi Arabia for approving, providing necessary facilities, and financial support by; Grant No 7716-PHM-2018-3-9-F.
REFERENCES