



|
Kola Nitida Leaf Phytoconstituents and Its Hepatoprotective, Antidiabetic, and Antihyperlipidemic Efficacy
Nadia M. Sokkar1,2, Basma G. Eid3, Zeinab T. Abd El Shakour4, Abeer Badiab1, Nagla A. El-Shitany3,5* |
|
1 Department of Natural Products, Faculty of Pharmacy, King Abdulaziz University, Saudi Arabia. 2 Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Egypt. 3 Department of Pharmacology & Toxicology, Faculty of Pharmacy, King Abdulaziz University, Saudi Arabia. 4 Laboratory of phytochemistry, National Organization for Drug Control and Research, Egypt. 5 Department of Pharmacology and Toxicology, College of Pharmacy, Tanta University, Egypt. |
ABSTRACT
Introduction: Kola nitida (KN) is a tropical plant belonging to the familyMalvaceae. Aim: To explore the metabolic profile and health benefits of KN leaf. Methods: The active metabolites were employed by HPLC-UV-MS/MS. The hepatoprotective of the hydromethanolic KN leaf extract (KNLE) was tested against CCl4-induced hepatotoxicity in rats. Besides, KNLE antihyperglycemic and antihyperlipidemic properties were studied in streptozotocin (STZ)-induced diabetic rats. Results: Eight phenolics were detected quantitatively. KNLE (100 mg/kg) significantly reduced liver index, serum ALT, AST, ALP, total bilirubin, ACP, β-GAL, and β-NAG relative to CCL4. The biochemical results were corroborated with the liver histopathology. Diabetic rats pretreated with KNLE (200 mg/kg) significantly lowered serum glucose, HbA1c, triglyceride, total cholesterol, and very-low-density lipoproteins compared to diabetic rats. Conclusion: KNLE protected the liver against CCl4 induced hepatotoxicity compared to silymarin. Besides, KNLE exerted antihyperglycemic and antihyperlipidemic properties in diabetic rats compared to glibenclamide.
Key Words: Kola nitida, chromatography, hepatoprotective, antihyperglycemic, antihyperlipidemic
INTRODUCTION
Kola nitida (KN) (family Malvaceae) is a tropical plant commonly cultivated in northern Nigeria. It contains numerous phytochemicals, including xanthine alkaloids, polyphenols, and vitamins [1–4].
The use of Kola nuts for refreshing the mouth in chewing sticks is common in Nigeria and Sudan. Powdered seeds can be consumed as tonics and powerful treatment for diarrhea. KN is used interchangeably with Kola acuminata as they have similar therapeutic uses. Furthermore, other parts such as twigs, flowers, fruits follicles, and bark are chewed to cause central stimulation and alleviate overstrain and depression. They are also used as a non-addictive stimulant against dysentery, vomiting, headaches, migraine, tonics, diuretic, astringent, antidepressant, to cure chest complaints, and stomach ulcers [5–7]. The nuts may be exploited in several ways to develop new pharmaceuticals with antioxidant, antibacterial [8], antidiabetic [9], and anti-inflammatory effects [10]. Although there is an ample amount of reports investigating the edible nuts of KN, scarce data exist about the constituents and therapeutic potential of the leaf (considered a waste product).
This study aimed to assess the phytoconstituents of the Nigerian species of KN leaf by HPLC-UV-MS/MS. Furthermore, the study aimed to evaluate its hepatoprotective, antihyperglycemic, and antihyperlipidemic potentials.
METHODOLOGY
Plant material
Identification of the KN branches was performed at Benin National Herbarium, Abomey-Calavi University, after collection from Adjarra village, Benin, Nigeria. KN leaves were dried at 25 ± 2 °C for 12 days, milled, and kept frozen. A voucher specimen (K-352) was deposited at the Department of Natural Products Herbarium, Faculty of Pharmacy, KAU.
Chemicals
HPLC grade solvents, Merck, Germany; veratric acid, protocatechuic acid, chlorogenic acid, and catechin standards, Sigma Chemical Co., USA; TLC pre-coated sheets with silica gel F254, Fluka, Germany; streptozotocin (STZ), Sigma-Aldrich, USA; glibenclamide (daonil 5 mg), Aventis, Egypt; and silymarin (instant), SEDICO, Egypt were used in this study.
Preparation of KN leaf extract (KNLE)
The hydromethanolic KNLE was obtained by agitating a mixture of500 g dried powdered leaves with 2 L of 70% methanol for 6 h then left for 24 h (n = 3). The combined extract was filtered and concentrated using the rotary evaporator (50 °C). The sticky residue was used in TLC and biological experiments.
KN powder (5 g) was defatted with light petroleum (10 h). The dried marc was extracted with 70% methanol in a sonicator (3 h, 25 °C), then filtered and concentrated. The residue was dissolved in methanol (HPLC grade) to a concentration of 10 mg/ml then filtered by a syringe-filter-membrane (0.45 µm) before injection.
General equipment
A mass detector (Thermo ion trap-mass spectrophotometer LCQ Advantage Max, Thermo Finnigan, USA) was used. The settings were: temperature, 400 ˚ C; capillary voltage, 4 kV; nitrogen as nebulizing gas (pressure, 65 psi; flow, 11 l/ min). The capillary heat, 350 °C. m/z 90 - m/z 2000 was the scanned mass covered; collision gas, He; a Hamilton syringe pump (flow rate 10 μl / ml) connected to the electrospray ionization was used for full scan mass infusion. Total Ion Mapping (TIM) experiment was the LC-MS/MS technique used.
HPLC analysis was performed at 25 °C using UV detector (330 nm); 20 μl sample; Agilent Zorbax Extend-C18 column (4.6 x 150 mm, 5 µm). The HPLC pump was programmed to deliver 70% acetonitrile: 30% aqueous formic acid (0.05%, v/v). A flow rate of 0.5 ml/min was adopted.
HPLC-UV-LC-MS/MS quantification of the phenolics
The external standards are prepared at serial concentrations in methanol and kept frozen. Before injection, the standard solutions were filtered (0.45 μm). Three determinations were recorded for each concentration. Quantitative analysis was determined by recording the peak area compared with 6 concentrations of each standard and to plot standard curves (n = 3). Identification of veratric acid was carried out as veratric acid; protocatechuic acid hexoside as protocatechuic acid; procyanidin B1, procyanidin B2, catechin, epicatechin as catechin; neochlorogenic and chlorogenic acids as chlorogenic acid. The sample solutions were injected 6 times to determine the precision test. The values of inter- and intra-day variability were utilized to assess the repeatability. To assess inter-day reproducibility, the protocol was carried out on 3 separate days. Samples were analyzed 3 times in one day to determine the intra-day variability. Precision was measured using the relative standard deviation (RSD).
Biological screening experiments
The experiments were approved by the Research Ethics Committee, Faculty of Pharmacy, KAU (PH-113-41).
Assessment of KNLE hepatoprotective efficacy against CCl4-induced hepatotoxicity
Thirty-two male Wistar rats (250-300 g) were placed into 4 groups (n = 8). The hepatotoxicity was induced in groups 2, 3, and 4 by oral administration of CCl4 in olive oil (0.5 ml/kg 3 times weekly for 4 weeks) [11]. Control: olive oil; CCl4; KNLE+ CCl4: oral 100 mg/kg KNLEdaily [12]; and silymarin + CCl4: oral 50 mg/kg silymarin daily. After 4 weeks, blood was withdrawn from the retro-orbital plexus and centrifuged at 3000 rpm for serum separation. Rats were decapitated, and the livers were removed and weighed. One part of the liver was stored in 10% formalin for the histopathology. Another part of the liver was kept frozen at -80 °C for the lysosomal fraction separation. The liver index was determined using the following formula [13]:
Liver Index= Liver Weight Body Weight×100
The synthetic liver function was assessed by measuring serum ALT, AST, ALP, and TP using locally available kits. Besides, the liver secretory function was evaluated by measuring TB using the kits of Crescent Diagnostics, Saudi Arabia.
Hepatic lysosomal membrane permeability was assessed by measuring the activities of ACP, β-GAL, and β-NAG.
For the liver histopathology, H & E stained sections were examined and photographed in a blind manner using light microscopy.
Assessment of KNLE antidiabetic and antihyperlipidemic efficacy against STZ-induced diabetes
Fifty-six male Wistar rats (150 ± 20 g) were divided into 7 groups (n = 8). Diabetes was induced in group 4-7 by intraperitoneal injection of STZ (50 mg/kg) freshly prepared in citrate buffer solution (pH 4.5, 0.1 M) (Abdel-Sattar et al., 2017). Control: citrate buffer; KNLE (100 mg/kg): 100 mg/kg KNLE; KNLE(200 mg/kg): 200 mg/kg KNLE; STZ; STZ + KNLE(100 mg/kg): diabetic rats received 100 mg/kg KNLE; STZ+ KNLE(200 mg/kg): diabetic rats received 200 mg/kg KNLE; and STZ + glibenclamide: diabetic rats received 5 mg/kg glibenclamide [14]. All treatments were orally for 4 weeks.
For assessment of the oral glucose tolerance test (OGTT), rats were orally administered 50% glucose solution (2 g/kg), and blood glucose levels were measured at 0, 30, 60, 90, and 120 min using a glucometer (ACCU-CHEK®, Roche, Switzerland).
Bodyweight gain was determined using the following formula:
Bodyweight gain =Final body weight-Initial body weight
Fasting blood samples were withdrawn to separate the serum. Serum glucose, insulin, and HbA1c were assayed using the kits of Reactivos GPL (Barcelona, Spain), Immunospec (Canada), and Spectrum-diagnostics Immuno turbidimetry (Egypt). Furthermore, serum TG, TC, HDL, LDL, and VLDL were assayed using Biodiagnostic Egypt kits.
Statistics
Values are presented as mean ± SE. ANOVA followed by Turkey’s HSD was applied via GraphPad prism to compare the significance between treatments at P ≤ 0.05.
RESULTS
HPLC analysis
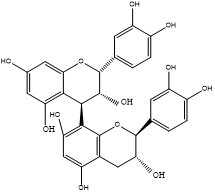
Results of LC-MSn (n=2) (Table 1, Figures 1 and 2) in a negative mode and at a collision energy of 35 eV. Peaks appeared at Rt 2.8, 4.2 for veratric acid (1), and protocatechuic acid hexoside (2) with [M–H] − at m/z 181 and 315, respectively. Chlorogenic acids, key information was provided by the fragmentation patterns of the isolated pseudo molecular ions to characterize isomeric compounds. A deprotonated ion at m/z 353 of a mono caffeoylquinic acid, and other characteristic major peaks at m/z 191, m/z 173, and m/z 179 for quinic acid, dehydrated quinic acid, and caffeic acid, respectively, as prominent fragments noted in the ions’ spectra. This fragmentation is characteristic of 3-O-caffeoylquinic (5) and 5-O-caffeoylquinic (7) acids. An intense caffeic acid ion at m/z 179 for 3-O-caffeoylquinic acid (with an intensity of ~ 50% compared with the tiny peek of the 5-O-caffeoylquinic acid) aided in their differentiation. Four flavan‑3‑ols were identified as the dimer procyanidins appeared at Rt 8.8 and 9.1 with [M–H]− at m/z 577 are determined as procyanidin B1 (3), and procyanidin B2 (4), respectively, as well as the two stereoisomers catechin (6) and epicatechin (8), appeared at Rt 11.8 and 14.7, respectively. The identification was based on comparison with previous work [15, 16], NIST database, and standards.
Table 1:Phenolics in the defatted KNLE
|
Peak no. |
Name |
RT |
MW |
Parent ion |
[m/z], MS2 |
|
|
Veratric acid |
2.8 |
180 |
181 |
163, 148, 101 |
|
|
Protocatechuic acid hexoside |
4.2 |
316 |
315 |
153, 135,109 |
|
|
Procyanidin B1 |
8.8 |
577 |
576 |
563, 504, 429, 425, 407 |
|
|
Procyanidin B2 |
9.1 |
577 |
576 |
553, 528, 451, 425, 407 |
|
|
3-O-Caffeoylquinic acid |
10.0 |
354 |
353.2 |
191,179, 173, 135 |
|
|
Catechin |
11.8 |
290 |
289 |
271, 245, 205, 179, 125 |
|
|
5-O-Caffeoylquinic acid |
13.2 |
354 |
353 |
191, 179, 173, 135 |
|
|
Epicatechin |
14.7 |
290 |
289 |
271, 245, 205, 179, 137 |
Validation data
The standards veratric, protocatechuic and chlorogenic acids and catechin showed good linearity r2 = 0.963, 0.999, 0.998, and 0.955 respectively; the inter-day precision revealed RSD of 1, 1.58, 1.65, 2.33%, respectively and intra-day variations of 1.01, 1.11, 2.02, 2.27%, respectively. The average regression equations: y = 99.076x - 68.421, R² = 0.8581 (veratric acid); y = 155.85x - 512.11, R² = 0.9933 (protocatechuic acid), y = 110.69x - 479.23, R² = 0.9362, (chlorogenic acid); y = 104.31x - 27.271, R² = 0.9834 (catechin). A range of 99.78% to 97.34 % was obtained for their recovery tests with RSD less than 2%. The detection limit values of veratric acid, protocatechuic acid, chlorogenic acid, and catechin were 0.21, 0.13, 0.22, and 0.11 μg/ml, respectively. The determination limits were 0.63 and 0.39, 0.66, and 0.33 μg/ml, respectively. The quantitative investigation was calculated in mg 100 g/powder plant ± SD and recorded a percentage of veratric acid (1.95 ± 0.0321), protocatechuic acid hexoside (0.7 ± 0.0332), catechin (5.46 ± 0.0031), epicatechin (4.53 ± 0.0240), neochlorogenic acid (3.54 ± 0.2421), chlorogenic acid (1.72 ± 0.0022), procyanidin B1 (1.33 ± 0.0044), and procyanidin B2 (1.03 ± 0.0021).
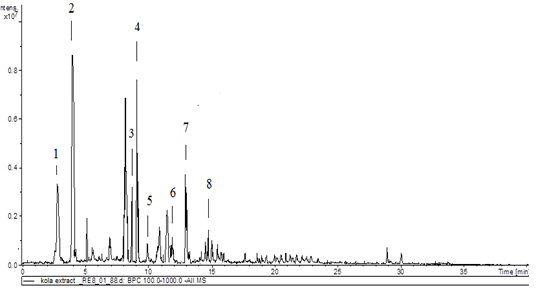
Figure 1: HPLC chromatogram of KNLE
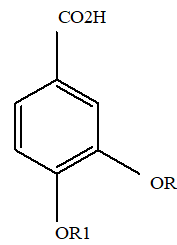
R R13
1 C H3 C H3
2 H H
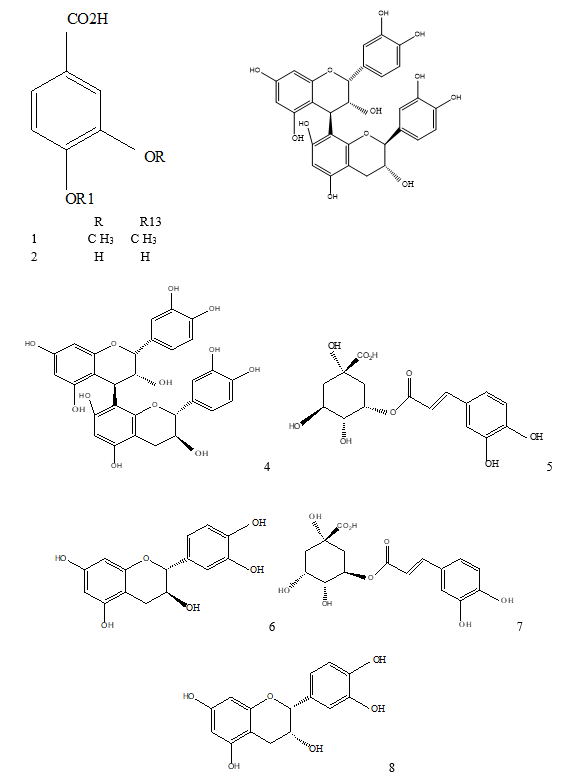
Figure 2: 1: Veratric acid; 2: protocatechuic acid; 3: procyanidin B1; 4: procyanidin B2; 5: neochlorogenic acid; 6: catechin; 7: chlorogenic acid; 8: epicatechin
Hepatoprotective efficacy
KNLE significantly decreased liver index, serum ALT, AST, ALP, and TB relative to CCL4. The effect of KNLE was like silymarin (Table 2 and Figure 3). Furthermore, KNLE significantly decreased liver lysosomal enzymes ACP, β-GAL, and β-NAG compared to CCL4. The effect of silymarin was significant compared to KNLE (Table 2).
Histopathological findings confirmed the hepatoprotection of KNLE against CCl4. Sections from the liver of rats treated with KNLE showed minimal damage and distinct preservation of hepatocytes structure and architecture, the effects that were also comparable to that of silymarin (Figure 4).
Table 2: Effect of KNLE on liver function
|
Parameters |
Groups |
|||
|
Control |
CCl4 |
KNLE+ CCl4 |
Silymarin + CCl4 |
|
|
ALT (U/L) |
27.4 ± 1.02 |
49.2 ± 1.21 a |
31.0 ± 1.52 b |
28.6 ± 1.15 b |
|
AST (U/L) |
53.8 ± 2.28 |
127.1 ± 3.07 a |
73.6 ± 2.89 b |
71.4 ± 4.09 b |
|
ALP (U/L) |
222.5 ± 11.5 |
568.5 ± 30.9 a |
310.0 ± 21.4 b |
306.9 ± 19.1 b |
|
TP (mg/dl) |
6.49 ± 0.15 |
4.93 ± 0.05 a |
5.12 ± 0.21 |
5.72 ± 0.26 |
|
TB (mg/dl) |
0.72 ± 0.02 |
1.66 ± 0.03 a |
0.94 ± 0.02 b |
0.88 ± 0.05 b |
|
ACP (U/L) |
130.5 ± 5.15 |
310.0 ± 15.0 a |
228.2 ± 5.61 b |
156.2 ± 14.2 b, c |
|
β-GAL (U/L) |
35.8 ± 4.5 |
94.2 ± 4.53 a |
50.8 ± 2.16 b |
39.5 ± 1.55 b, c |
|
β-NAG (U/L) |
30.5 ± 1.64 |
83.8 ± 3.73 a |
54.2 ± 1.18 b |
41.0 ± 2.08 b, c |
Values are presented as mean ± SE. a Significant from control, b CCl4, cKNLE + CCl4
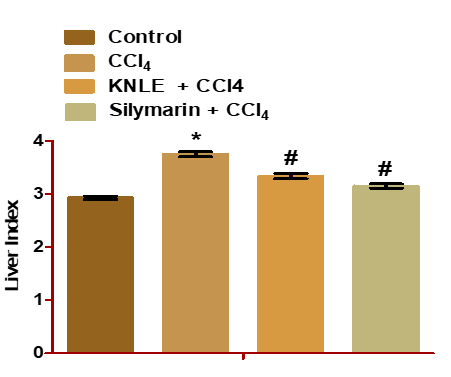
Figure 3: Effect of KNLE on the liver index. The values are presented as mean ± SE. * Significant from control, #CCl4
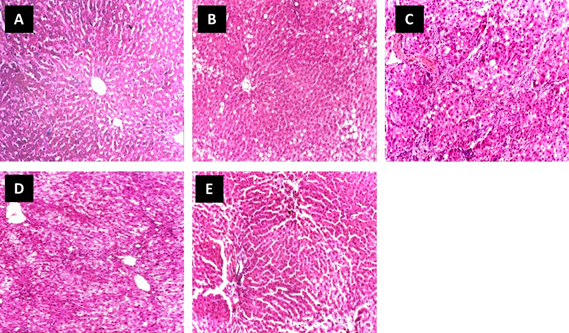
Figure 4: Effect of KNLE on liver histopathology examined in CCl4 rats (H & E x 100). Photo [A]: control showing hepatocytes cords radiating from the central vein. Photo [B and C]: CCl4 showing fatty degeneration, necrotic areas, and portal loss of architecture with mild congestion of blood vessels. [D]: KNLE + CCl4 showing minimal damage, distinct hepatocyte structure, and architectural preservation. [E]: silymarin + CCl4 showing normal hepatocytes and liver lobules architecture
Antidiabetic and antihyperlipidemic efficacy
In OGTT, KNLE100 mg/kg significantly lowered blood glucose of the diabetic rats at 60, 90, and 120 min compared to STZ. KNLE200 mg/kg significantly lowered blood glucose of the diabetic rats at 0, 30, 60, 90, and 120 min compared to STZ. KNLE 200 mg/kg significantly lowered glucose compared to KNLE 100 mg/kg. Moreover, glibenclamide significantly lowered glucose compared to KNLE 200 mg/kg (Figure 5).
KNLE significantly decreased serum glucose and HbA1c compared to STZ. Concerning HbA1c, KNLE was as effective as glibenclamide, while glibenclamide outperformed KNLE in reducing glucose. Both treatments exerted no effect on STZ-induced bodyweight loss and insulin reduction (Figure 6).
KNLE significantly decreased serum TG, TC, and VLDL compared to STZ. KNLE significantly decreased TG of the normal rats. Both KNLE and glibenclamide equally lowered TG. Moreover, glibenclamide significantly lowered TC, LDL, and VLDL compared to KNLE. Both treatments exerted no effect on STZ-induced HDL reduction (Figure 7).
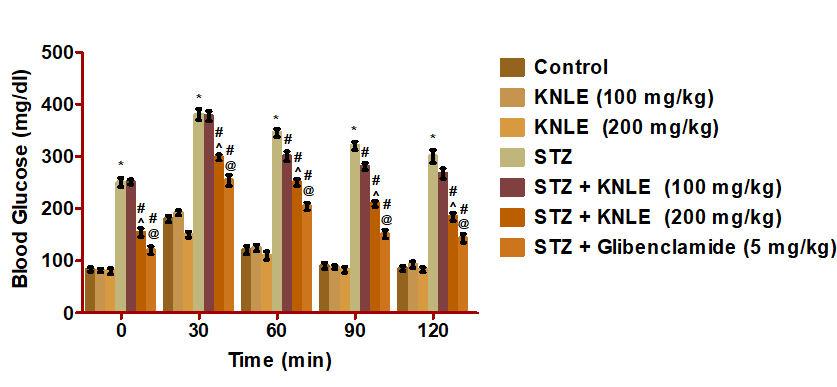
Figure 5: Effect of KNLE and glibenclamide on oral glucose tolerance test. Values are presented as mean ± SE. * Significant from control, #STZ,^ STZ + KNLE (100 mg/kg); @STZ + KNLE (200 mg/kg)
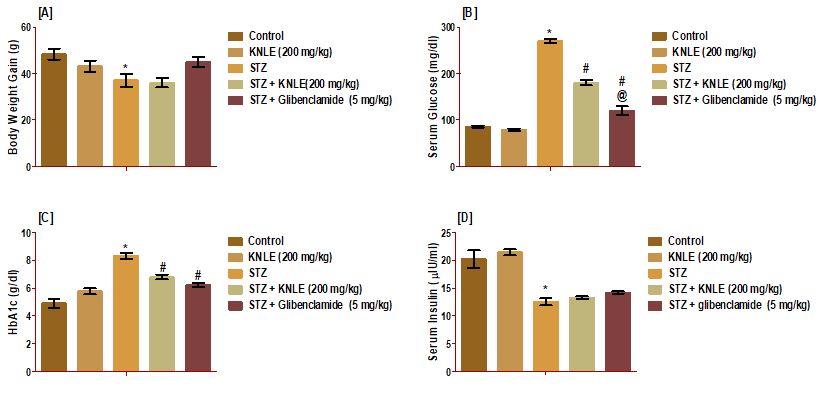
Figure 6: Effect of KNLE and glibenclamide on A: body weight gain; B: serum glucose; C: HbA1c; D: serum insulin. Values are presented as mean ± SE. * Significant from control, #STZ;@ STZ + KNLE (200 mg/kg)
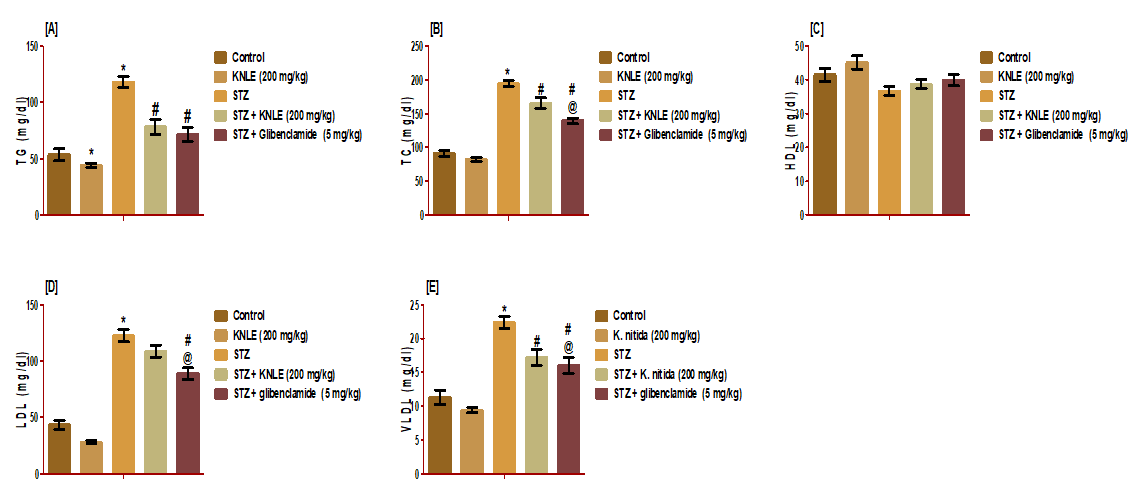
Figure 7: Effect of KNLE and glibenclamide on serum lipid profile A: TG; B: TC; C: HDL; D: LDL; E: VLDL. Values are presented as mean ± SE. * Significant from control, #STZ;@ STZ + KNLE (200 mg/kg)
DISCUSSION
CCl4 caused an acute liver injury after metabolism by CYP2E1 enzyme in the endoplasmic reticulum. This reaction generated a highly toxic trichloromethyl radical, which further forms trichloromethyl peroxy radical after reacting with oxygen [17]. These free radicals are thought to disturb Ca2+ homeostasis by stimulating lipid peroxidation when reacting with polyunsaturated fatty acids present in the endoplasmic reticulum [18, 19]. This reaction causes a loss of cell membrane integrity and causes the cell to release liver enzymes. Elevation in TB level may be a result of intrahepatic canaliculi obstruction produced by the inflammation of the hepatic cells. On the other hand, TP levels are decreased, perhaps due to damage in the endoplasmic reticulum due to loss of P450 and a halt of protein synthesis [20, 21]. The KNLE provided a significant hepatoprotective effect that was comparable to the effect of silymarin. Increased protein levels indicate that the endoplasmic reticulum was stable, protein synthesis, as well as regeneration of hepatocytes, had been enhanced [22]. Besides, ALP levels dropped, suggesting a stable function of the biliary system.
It has been shown that lysosomal enzymes, which are released intracellularly preceding cellular death, initiates cellular injury, and causes necrosis. This was indicated by the increase in the extracellular lysosomal enzymes due to CCl4 toxicity [23]. However, treatment with KNLE antagonizes such an effect. This may occur due to the potential stabilizing effect of polyphenols and phenolic acids, which heal the hepatic parenchyma and lead to hepatocyte regeneration.
A weight reduction caused by STZ is likely caused by injury to the β-cells of islets of Langerhans accompanied by the release of more free fatty acids by the adipose tissue. This arises due to decreased insulin levels, which caused excessive production of LDL-cholesterol [24]. This effect was not reversed after treatment with either KNLE or silymarin. In diabetic rats, loss in body weight is expected due to some disturbances in carbohydrate metabolism, such as glycogenolysis, lipolysis, acidosis, and protein deficiency [25]. HbA1c gives an indication of the average levels of blood sugar over the recent past. According to WHO [26] value, less than 6.5% does not exclude diabetes diagnosed using glucose tests. In the present study, diabetic rats had shown a level of HbA1c higher than that recommended by WHO or the level in normal rats. This was improved after treatment by KNLE (200 mg/kg) or glibenclamide. This positive response may refer to reduced intestinal glucose absorption and improved lipid and carbohydrate metabolism. Administration of KNLE (200 mg/kg) improved glucose tolerance, and the lipid profile (TG, TC, and VLDL). This is related to the polyphenols content of KNLE previously discussed in scientific reports [27, 28].
CONCLUSION
KN leaf imported from Nigeria was mostly considered as a waste; this study gave an overview of its biological properties as well as its unique phytochemical composition. To the best of our knowledge, this study provided an unprecedented report of phenolic composition and biological activity of the KN leaf. This leaf could potentially be exploited by the pharmaceutical industry due to its rich source of phytochemical entities.
Conflict of interest
No conflict of interest.
Financial resources
This work was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (166-160-D1440). The authors acknowledge the technical and financial support from DSR.
REFERENCES