



|
Influence of Milk Quality and Production Factors on the Presence and Amount of Histamine in Traditional Yogurt
M. Jahedinia1, G. Karim2*, I. Sohrabi Haghdust3, S.M. Razavi Rohani4 |
|
1 Ph.D. student, department of food hygiene, Science and Research Branch, Islamic Azad University, Tehran, Iran. 2Professor, Department of Food Hygiene, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran. 3Professor, Department of Pathology, Science and Research Branch, Islamic Azad University, Tehran, Iran. 4Professor, Department of Food Hygiene, Faculty of Veterinary Medicine, University of Urmia, Urmia, Iran. |
ABSTRACT
Biogenic amines are organic, basic nitrogenous compounds of low molecular weight that are mainly generated by the enzymatic decarboxylation of amino acids by microorganisms. Histamine is the only BA with a defined legal upper limit in foods (5mg100g-1) and the most frequent foodborne intoxications and intolerances, caused by biogenic amines, involve histamine. Dairy products are among the foods with the highest amine content. Yogurt consists of approximately 60 percent of the per capita dairy consumption of Iranian people. In this study, the effect of milk quality (subclinical mastitis) and production factors including starter content, storage time, and temperature on the presence and amount of histamine in yogurt were determined. A reversed-phase HPLC method was employed for histamine determination. The mobile phase consisted of acetonitrile/water (18: 82) and the flow rate was 0.5 ml min-1using isocratic HPLC. Detection with UV detector was carried out at 254nm. Histamine concentration was different in yogurt samples and below the acceptable limit. Analysis of data using multivariable regression showed that there is a significant correlation between histamine amount and all of the studied factors except starter content. The results showed that amounts of histamine in yogurt cannot cause a health hazard and it is possible to reduce the accumulation of histamine in yogurt by improvement in the production process and milk quality.
Key Words: Histamine, Traditional yogurt, HPLC.
INTRODUCTION
Biogenic amines are organic, basic nitrogenous compounds with biological activity [1, 2] that are mainly produced by microorganisms with decarboxylase activity [3]. They can cause adverse effects in humans when eaten in large amounts [4]. Histamine, putrescine, cadaverine, tyramine, tryptamine, β-phenylethylamine, spermine, and spermidine are the most important biogenic amines in foods [5].
They can be formed in food during processing or the period of storage [6]. The prerequisites for biogenic amine formation by microorganisms are (i) availability of free amino acids, even (ii) presence of decarboxylase-positive microorganisms; and, (iii) conditions that allow bacterial growth [7]. Histamine is the only BA with a defined legal upper limit in foods (5mg 100g-1) [8]. Because of the most frequent foodborne intoxications and intolerances, caused by biogenic amines, related to histamine [1]. Ingestion of foods that contain high levels of histamine cause rash, urticaria, edema, localized inflammation, nausea, vomiting, diarrhea, hypotension, headache, palpitations, tingling, burning, and itching [4], histamine intolerance, arises when histamine degradation is impaired [9], occurs especially in diamine oxidase (DAO)-deficient subjects [10]. Dairy products, especially cheeses, are one of the foods with the highest amine content and are examples to increase of histamine content during improper food processing [11, 12]. Several outbreaks of histamine poisoning have occurred after the consumption of cheese containing high levels of histamine [13] Yogurt a nutrient-dense probiotic food is one of the most popular fermented milk products worldwide [14, 15].
It consists of approximately 60 percent of dairy product consumption of Iranian people [16]. Because of the different climate in Iran, there is a wide variety of traditional dairy products in rural areas. The Iranian people prefer to consume traditional dairy products due to their great natural tastes and flavors [17].
Many strains of lactic acid bacteria have histidine decarboxylase activity .the researches showed most of Streptococcus thermophilus and Lactobacillus bulgaricus isolates that are the main starter of yogurt can produce histamine too [6, 10, 18, 19] so histamine may be accumulated in traditional yogurt that contains varied and uncontrolled starter.
The presence and accumulation of biogenic amines depend on many factors such as availability of free amino acids (level of proteolysis), pH, water activity, salt-in-moisture level, storage time and temperature, bacterial density, and hygienic condition. [20, 21].
This study aimed to determine the effect of milk quality and production factors on the presence and amount of histamine in traditional yogurt.
MATERIALS AND METHODS
Milk samples: 3 raw cow's milk samples were taken from the bulk milk tank of 3 different dairy farms. SCC (Somatic Cell Count) of each sample was measured by automatic SCC counter (DELAVAL Sweden) and physical and chemical factors were measured by ultrasonic milk analyzer (Ekomilk, Eon Trading, Bullgary).
Plan of an experiment: In this study effect of milk quality (Somatic cell count) and production factors including starter content (2%, 3%), storage time (10, 20, 30 days) and temperature (4, 10 °C) on the presence and amount of histamine in traditional yogurt was determined.
3 whole milk samples with 3 different range of SCC were used for yogurt making. Traditional starter culture (Chenaran Region of North Khorasan Province, Iran) were used for the production of yogurt and were added in 2 different levels to milk samples, so 6 samples of inoculated milk Were prepared that were incubated at 42°C until PH 4.2 was reached. First Series were stored at 4°C and the second series was stored at 10°C for 30 days. Physicochemical analyses and Microbial tests were done on days10, 20, and 30 after production.
The relationship between variables was determined using multivariable regression.
Preparation of yogurt: Milk samples heated at 90°C for 10 min and then cooled to 43°C. 3 whole milk samples with 3 different SCC (low, intermediate, high) were used for yogurt making. Traditional starter culture (Chenaran Region of North Khorasan Province, Iran) was used for the production of yogurt and were added in 2 different levels (2 and 3%) to milk samples that were packed in polyethylene containers, so 6 samples of inoculated milk Were prepared that were incubated at 42°C until PH 4.2 was reached. The first series was stored at 4°C and the second series was stored at 10°C for 30 days.
Statistical analyses:
The relationship between amounts of histamine as a dependent variable and the independent variables including SCC, starter content, storage time, and temperature were assessed by multivariable regression. Statistical analysis of the data was performed by using SPSS version 21 for windows. A p-value below 0.05 was regarded as significant
Histamine determination
Histamine amount of samples was measured on days10, 20, and 30 after production.
Instruments: The chromatographic system consisted of a Wellchrom HPLC pump, K-1001 (KNAVER Germany), dynamic mixing chamber, degasser (KNAVER), Wellchrom solvent organizer K-1500 (KNAVER Germany), UV-detector K-2501 (KNAVER Germany), and a personal computer running the software Eurochrom 2000. The column was Eurospher 250/4.6 C18 Gravity 5 μm with precolumn
Chromatographic conditions: A reversed-phase Isocratic HPLC method was employed for histamine determination. The mobile phase consisted of acetonitrile and water (18:82 v/v) and the flow-rate was 0.5 ml min-1. The peaks were detected at 254 nm.
Sample preparation: Samples were prepared by acid extraction and derivatization by the method of Aliakbarlu et al. 2009
20 ml of 0.1 M HCl was mixed to 10 g of yogurt sample and then homogenized for 2 min using a disintegrator Heidolph Diax 900 (Heidolph Instruments GmbH, Kelheim, Germany). The suspension was centrifuged at 12000 g for 20 min at 4°C. The supernatant was removed and the above step was repeated for solid phase. 2 liquid phases were mixed and 0.1 M HCl was added to 50 mL and filtered. Then 5 mL of acid extract was mixed with 5 mL butanol 3 times. The organic extracts were saturated with NaCl and pH was reached to 11.5 with NaOH.
For the derivatization of the samples, 1 mL organic extract was mixed with 2 drops of 1 M HCl and dried using vacuum oven (LABOROTA 4003 Heidolph instruments). Then 1 mL of 0.1 M HCl, 500 μL saturated solution of NaHCO3, and 1 ml dansyl chloride solution (5 mg mL-1) were added. Samples incubated at 40°C for 1 h, then the solution was dried under vacuum and 2 mL acetonitrile was added. The solution was filtered (VARIAN, Bond Elut C18) and injected onto the chromatographic column.
RESULT
Statistical analysis of histamine values in days 10, 20 and 30 after production
The independent variables include SCC, starter content, storage temperature, and dependent variables are the concentration of histamine in days 10, 20, and 30 after production. The effect of independent variables was assessed by multivariable regression. according to Table 1 Multiple correlation coefficient (R) for the amount of histamine in days 10, 20 and 30 is0.967, 0.969 and0.965 respectively that shows there is a high correlation between independent and dependent variables.
Table 1: Summary of the regression model for histamine concentration
|
Model Summary |
|||||||||
|
Model |
R |
R Square |
Adjusted R Square |
Std. Error of the Estimate |
Change Statistics |
||||
|
|
|
|
|
|
R Square Change |
F Change |
df1 |
df2 |
Sig. F Change |
|
1: Histamine concentration (day 10) |
0.967 a |
00.936 |
0.922 |
0.55420 |
0.936 |
69.122 |
4 |
19 |
0.000 |
|
2: Histamine concentration (day 20) |
0.969a |
0.939 |
0.927 |
0.69014 |
0.939 |
73.670 |
4 |
19 |
0.000 |
|
3: Histamine concentration (day 30) |
0.965a |
0.932 |
0.917 |
0.91097 |
0.932 |
64.887 |
4 |
19 |
0.000 |
One-way analysis of variance (ANOVA) showed that the F test result is significant (P=0.000) that confirm the regression model of study. It means all of the independent variables together including SCC, starter content, storage temperature can explain changes in histamine content and have a significant effect on histamine concentration in days10, 20, and 30 after production.
Beta Standardized Coefficients test showed that the highest coefficient belongs to storage temperature, SCC, and starter content respectively for histamine Concentration in days 10 and 20. It means temperature storage has the most contribution in explaining dependent variable variance. On day 30 SCC had the highest Beta Standardized Coefficients. According to table 2, all independent variable is at the significant level except starter content that shows this variable cannot explain the dependent variable that is histamine content in days 10, 20 and 30 after production.
The partial correlation coefficient in Table 2 shows the intensity of correlation with the dependent variable
Table 2: Coefficients for histamine concentration in days 10, 20 and 30
|
Model |
Histamine concentration (day 10) |
Histamine concentration (day 20) |
Histamine concentration (day 30) |
||||||
|
Beta Standardized Coefficient |
Significance |
Partial Correlations |
Beta Standardized Coefficient |
Significance |
Partial Correlations |
Beta Standardized Coefficient |
Significance |
Partial Correlations |
|
|
Somatic cell count |
0.253 |
0.000 |
0.707 |
0.290 |
0.000 |
0.762 |
0.320 |
0.000 |
0.775 |
|
temperature |
0.306 |
0.000 |
0.770 |
0.326 |
0.000 |
0.799 |
0.301 |
0.000 |
0.756 |
|
Starter content |
0.147 |
0.274 |
0.250 |
0.166 |
0.204 |
0.289 |
0.168 |
0.224 |
0.277 |
Statistical analysis of storage time effect on the histamine content
Effect of Storage Time on the histamine content in days 10, 20, and 30 after production was studied by repeated-measures analysis of variance.
Mauchly's Sphericity test and Greenhouse-Geisser test were significant for storage time (P=0.000) that showed histamine concentration had a significant difference in repeated measurements (days 10, 20, and 30). The value of Partial Eta was 0.684 so the effect of storage time on histamine content is average.
All of P-values in Pairwise comparison analysis were below 0.05 (P=0.000) that proved histamine concentration had a significant difference during the time and had a significant increase during storage time.
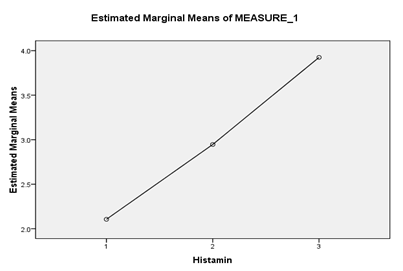
Figure 1: Estimated Marginal Means for histamine concentration in days 10, 20 and 30
DISCUSSION
Biogenic amines production is a complex process and depends on several factors [22]. Milk is a proteinaceous food so Dairy products, are susceptible to biogenic amine production [4].
biogenic amine production is strain-dependent [6, 23] and some strains of lactic acid bacteria are the main producer of histamine in the dairy product [24]. Researches confirmed that some strains of Streptococcus thermophilus, lactobacillus buchneri, and Lactobacillus delbrueckii subsp. Bulgaricus have histidine decarboxylase activity [10, 18, 25]. Since traditional yogurt different strains of lactic acid bacteria can be present, histamine can be produced in high concentrations.
Greater knowledge of the factors involved in the synthesis and accumulation of BA should lead to a reduction in their incidence in dairy products [24]. The most important procedures are improving the sanitary condition during production and storage and selection of proper strain as starter cultures [26, 27].
In this study, the effect of milk quality (subclinical mastitis) and production factors including starter content and storage time and temperature on the presence and amount of histamine in traditional yogurt were determined.
The results showed that histamine can produce in traditional yogurt at different levels depend on milk quality and production and storage factors. The maximum concentration of histamine was 10.89±0.78 mg kg-1 that is below the maximum limit of the FDA. Different studies reported different values for histamine concentration in yogurt may be because of various strains of starter culture available in yogurt.
Leszczyńska et al (2004) determined the histamine content in some samples of food products [28]. The histamine concentrations in 4 yogurt samples were measured by Eliza method was 0.50±0.03, 0.078±0.04, 0.29±0.03and 0.039±0.02 mg 100g-1
Min et al (2004) examined the levels of biogenic amines in foods of animal origin distributed on Korean domestic markets [29]. The histamine content of 4 yogurt samples obtained by the HPLC method was 1.81, 1.91, 21.2 and 15.8 µg g-1
Qin et al (2012) determined histamine in fermented food including yogurt [30]. The average concentration of histamine was 312.15 mg kg-1 in yogurt samples.
Costa et al (2015) reported values of 17.97 and 53.85 mg kg-1 in probiotic cows and goat's fermented kinds of milk after 10 days of storage [31].
In most developed dairy countries, milk quality is defined by the somatic cell count (SCC) in bulk tank milk. The SCC of milk is influenced by mastitis [32]. Hachana and paape (2012) proved that High SCC in milk increased proteolysis and lipolysis in yogurt during storage [33]. Sumner et al (1990) showed that histamine production is related to the level of proteolysis [34]. Results of statistical analysis in this study showed that there is a significant relationship between SCC and histamine content of yogurt samples.
Statistical analysis about the effect of storage temperature (4, 10°C) on histamine production showed that histamine in yogurt samples stored in 10°C was significantly higher than samples stored in 4°C
Several studies show that low temperatures decrease the accumulation of BA such as histamine although the accumulation of histamine above the maximum permissible limit may happen even in cheese is kept in refrigeration [24].
Calles-Enríquez et al (2010) inoculated a histamine-producing Streptococcus thermophilus strain in milk and store it in temperature (42 & 4°C) for 24 h [35]. They observed that there were no differences in hdcA expression level and cell numbers but the histamine content at 42°C was 10 times higher than the culture kept at4°C.
Effect of starter content (2, 3%) on histamine concentration was studied too. The determination of coefficients showed that the starter content is not an insignificant level, unlike other independent variables. Aliakbarlu et al (2009) investigated the effect of starter content (1, 3%) on biogenic amines production in Iranian white brine cheese [3]. They found that in low ripening temperature, histamine concentration increase with increasing starter content while in high ripening temperature histamine concentration decrease with increasing starter content.
Storage time was another factor that was examined. The results showed that there is a significant relationship between histamine concentration in days 10, 20, and 30, and the effect of time is average. Partial Eta= 0.684
Proteolysis increase during storage time [36] on the other hand when histamine is formed do not destroy [37] so histamine content increase during storage time. [38]
The results demonstrated the majority of the histamine was formed in the first 10 days and there was an increase in histamine concentration in the days 20 and 30 too.
CONCLUSION
Generally, the results of this study show that histamine can be formed at different levels in traditional yogurt.
The amount of histamine in any of the samples does not exceed the maximum permissible limit of FDA that indicates the presence of histamine in yogurt is not a health concern. Although it is possible to minimize it by improving the production process and quality of raw milk. In the present study, just one traditional starter culture was used, since there is various type of traditional yogurt with different strains of lactic acid bacteria further studies are needed.
ACKNOWLEDGMENTS
The authors are thankful for the support of the veterinary head office of north Khorasan and the Islamic Azad University of Sabzevar.
REFERENCES