




The present research aimed at developing and evaluating a vesicular drug carrier system for topical delivery of Repaglinide to provide sustained drug delivery. Repaglinide ethosomes were formulated using the method of thin film hydration by soya lecithin and evaluated for percent entrapment efficiency, vesicle size, percent drug content, surface morphology, and in-vitro drug release. The ethosomes were prepared by varying the variables such as concentrations of soya lecithin and ethanol, while entrapment efficiency and drug release were the chosen responses. Ethosomal formulation was optimized using the 32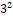 factorial design. The vesicle size of optimized batch was found to be 198.92 nm with zeta potential of -13.42 mV. The % entrapment efficiency was found to be 92.40 % and percent drug release of ethosomal gel was found to be 73.24%. Optical microscopic and scanning electron microscopic observations showed formation of spherically shaped vesicles. The in-vitro drug release of the formulation increases with increase in ethanol concentration and decrease in lipid concentration.
factorial design. The vesicle size of optimized batch was found to be 198.92 nm with zeta potential of -13.42 mV. The % entrapment efficiency was found to be 92.40 % and percent drug release of ethosomal gel was found to be 73.24%. Optical microscopic and scanning electron microscopic observations showed formation of spherically shaped vesicles. The in-vitro drug release of the formulation increases with increase in ethanol concentration and decrease in lipid concentration.
INTRODUCTION
The most extensive and easily accessible organ of the human body is skin. Skin as a means of medicine delivery may provide different benefits more than old drug delivery systems, such as lesser alterations in plasma medicine contents, prevention of gastrointestinal disorders and hepatic metabolism of the medicines, and high patient agreement [1]. It provides a superb obstacle to molecular transport, since stratum corneum is the most difficult obstacle to the passage of many medicines, except for low molecular weight and lipophilic medicines. For more efficiency of transdermal medicine delivery system, the medicine should be capable of penetrating the skin obstacle and reach the target region [2-4].
Ethosomes are the ethanolic phospholipid vesicular carriers which are soft, shapable vesicles utilized for medicines delivery to reach the deep skin layers or the systemic circulation. Ethosomes commonly use transdermal route for medicines delivery. Medicine can be entrapped in ethosomes that have different physicochemical properties such as amphiphilic, lipophilic, or hydrophilic. The ethosomal system comprises of phospholipid, high concentration of water and alcohol. The high content of ethanol makes ethosome unique. Ethanol results in disorder of skin fat bi-layer organization and so when incorporates into a vesicle membrane, it enhances the vesicle capability to penetrate the stratum corneum [5].
Repaglinide is of meglitinide class used to lower blood glucose level in type 2 diabetes mellitus. It decreases blood glucose level by stimulation of insulin release from the pancreas. Dosing frequency of repaglinide is 0.5-4 mg, 3- 4 times daily. It indicates quick start of action but for shorter period of action, as it is quickly eliminated from the blood stream with a half-life of nearly one hour. The mean definite bioavailability is approximately 56% due to its first pass metabolism [6, 7].
Repaglinide is a drug having high first pass metabolism and very short half-life, so there is requirement of frequent dosing for maintaining plasma concentration in body. Literature studies have shown the applications of ethosome as drug delivery system in providing sustained release and maintaining drug plasma drug concentrations in body. It may improve the bioavailability and thus help in decreasing the dosing frequency of the repaglinide and may also provide sustained release of drug. Thus, efforts were made to formulate the ethosome drug delivery system of repaglinide.
MATERIALS AND METHODS
Materials
Repaglinide was purchased from Swapnaroop Pharmaceutical Enterprises. Soya lecithin, cholesterol, chloroform, methanol, and ethanol were used of AR grade and were purchased from loba chemicals.
Methods
Preformulation studies
Preformulation studies were done to identify, characterize and confirm the purity of medicine and to study the adaptability between drug and excipients. The organoleptic properties of drug were evaluated. The melting point of drug was determined by capillary method and the absorption maxima of drug were determined using methanol as solvent. To study the drug excipient compatibility, FTIR and DSC studies were performed. FTIR scans were obtained over the wave number 4000 to 400 cm-1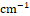 on FTIR-4100 spectrophotometer (Jasco Corporation, Japan). DSC studies were done for pure drug as well as drug and physical mixture (drug+cholesterol+lipid) in 1:1 ratio. Themograms were obtained through heating the samples at a fixed rate of 10°C/min. A dry purge of nitrogen gas was utilized for all runs. Specimens were heated from 20-300°C
on FTIR-4100 spectrophotometer (Jasco Corporation, Japan). DSC studies were done for pure drug as well as drug and physical mixture (drug+cholesterol+lipid) in 1:1 ratio. Themograms were obtained through heating the samples at a fixed rate of 10°C/min. A dry purge of nitrogen gas was utilized for all runs. Specimens were heated from 20-300°C
Preparation of repaglinide ethosomes using thin-film hydration
The ethosomal formulations were prepared using a thin-film hydration method. Repaglinide, soya lecithin and cholesterol were dissolved in chloroform and methanol in 2:1 ratio. The organic solvent was removed by rotary evaporator above the lipid transition temperature under vacuum. A homogeneous thin film was obtained on the wall of the flask. The resulting drug-lipid film was then rehydrated with distilled water and appropriate concentration of ethanol [8-11].
First, preliminary formulation of ethosomes was prepared using different concentrations of ethanol (20-40%), lipid (30-50 mg) and cholesterol (0-5 mg). The preliminary batches were evaluated and based on the results obtained after preliminary trials, design of experiment was applied for optimization of formulations. Full factorial design (32) was used to prepare repaglinide ethosomes and the parameters were evaluated and optimization was done. The independent variables selected for the study was concentration of lipid (X1) and concentration of ethanol (X2). The upper limit of X1 was 60mg and lower limit was 40mg whereas for X2 the upper concentration level was 35% and lower level was 25%.
Ethosomal gel formulation
A 1% w/w Carbopol 940 gel was prepared as vehicle for repaglanide ehosomes the ethosomal formulation was added to the hydrated carbopol solution, with stirring until a homogeneous ethosomal gel was obtained. pH of the gel was adjusted to neutral by triethanolamine and stirred slowly [9, 12].
Evaluation of ethosomes
Entrapment efficiency
Micro centrifugation technique was used to measure entrapment efficiency. The ethosomal sample was centrifuged at 1500 rpm for 15 min. After centrifugation the supernatant and pellets were separated and analyzed for the amount of drug by U.V. Spectrophotometer [6, 13].
Using the following equation, the entrapment efficiency was determined:
|
|
(1) |
Where, T = the theoretical amount of repaglinide that was added
C = amount of drug detected only in the supernatant
Vesicle shape
Ethosomal formulation was kept under optical microscope under 40X magnification to study vesicles shape.
Vesicle size
The optimized ethosomal formulation was analyzed for particle size and zeta potential by Nanophox zeta sizer.
Drug content
The drug content of ethosomal formulations was specified using UV spectrophotometer. The absorbance was recorded at 237nm the drug content was computed through the following formula [14]:
|
×100 |
(2) |
In-vitro drug release
In-vitro release studies on ethosomal gel preparation were carried out by Franz-diffusion cell. The cellophane membrane was soaked in 7.4 phosphate buffer for 24 hours. The cellophane membrane was placed between the donor and receptor compartment. A weighed amount of ethosomal gel preparation was put on one side of the cellophane membrane. The receptor medium was phosphate saline buffer of pH 7.4. The franz diffusion cell was placed on a magnetic stirrer with the stirring speed of 100 rpm and temperature was kept at 37±1°C. Specimens were withdrawn at specific time and analyzed spectrophotometrically at 237nm and cumulative drug diffusion was calculated [15].
32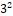 Factorial design
Factorial design
The prepared factorial batches of ethosomes were analyzed for their responses by using the Design Expert version 11 software (Trial version). Statistical significance of these factors was established with analysis of variance (ANOVA). The software specifies the major impacts of each parameter as well as the interactions between parameters by changing the contents of all parameters in parallel. The optimization feature can be applied to compute the optimal operating factors for a procedure.
Optimization of formulation
Optimization of the ethosomal gel was done by software using numerical analysis. The in range values of the entrapment and drug release was used for the calculation through software. The optimized formulation was evaluated for entrapment efficiency and in vitro drug release, and FTIR of the formulation was taken.
Scanning electron microscope
Vesicular shape and morphology of repaglinide ethosome was examined by scanning electron microscope. A thin film of the air-dried suspension or centrifuged vesicular portion was placed on a black carbon tape earlier mounted on the specimen chamber of the SEM. The specimen chamber was then closed and allowed to stay for some minutes while a vacuum was created. The electron accelerating voltage was then set at 20 KV and the instrument run. The vesicle sizes were simultaneously measured at different fields.
RESULTS AND DISCUSSION
Preformulation studies
The organoleptic property of drug was studied and it was found to be almost white crystalline powder with no characteristic odor. The melting point of repaglinide was found to be 1300C which is in accordance with the standard value. The drug was found to be practically insoluble in water and freely soluble in methylene chloride and methanol and it exhibited absorbance maxima at 237 nm in solvent methanol.
The FTIR spectrum recorded of drug was found in accordance with its chemical structure and showed characteristic peaks of N-H stretch at 3409 cm-1, N-H bend at 1564 cm-1 and C-H stretch at 2802cm-1 and O-H at 3308 cm-1. The physical mixture of drug with cholesterol and soya lecithin showed the characteristic bands of O-H at 3307 cm-1 and C-H stretch at 2929 cm-1. The spectra indicated retention of major peak with slight shift in the band indicating compatibility of drug with excipients.
The DSC thermogram of pure drug and physical mixture was carried out to study the purity and compatibility between drugs and excipient. The thermogram of pure drug showed endothermic peak at 136.80C confirming the purity of the sample and DSC thermogram of physical mixture showed the shift of the peak to 1200C. This shift to lower temperature could be due to the presence of lipid and cholesterol.
The evaluation studies of preliminary batches of repaglinide were performed. The entrapment efficiency of seven preliminary batches was found between 92.85 to 97.57%. The preliminary ethosomal batches showed percent drug release between 52.63% to 71.43%. Based on the preliminary studies the ethosomal formulations was optimized using design of experiment approach. 32 full factorial design was used for optimization and the effect of independent variables that is soya lecithin and ethanol at three levels were studied on entrapment efficiency and percent drug release.
Evaluations of 32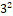 factorial batches of repaglinide ethosomal suspension and ethosomal gel
factorial batches of repaglinide ethosomal suspension and ethosomal gel
Nine batches of repaglinide ethosomal suspensions were prepared by thin film evaporation technique. The prepared factorial batches were evaluated for entrapment efficiency; percent drug release and vesicle size. The responses evaluated were statistically determined by using Statease Design Exert software and influence of independent variables were studied on the responses. The entrapment efficiency and vesicle size of factorial batches are listed in Table 1. The batch F8 exhibit highest entrapment efficiency 98.85% and batch F1 exhibits lower entrapment efficiency 87.53%.
The formulations of repaglinide ethosomal suspension were observed under the optical microscope for structural morphology. The spherical structure and lipid layer was observed. The optical microscope images are shown in Figure 1.
Table 1. Entrapment Efficiency of Repaglinide Suspension of 32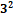 Factorial Batches
Factorial Batches
|
Formulation code |
% Entrapment efficiency |
Vesicle size (nm) |
|
F1 |
87.53 |
484.46 |
|
F2 |
91.26 |
385.65 |
|
F3 |
96.36 |
405.21 |
|
F4 |
92.66 |
299.62 |
|
F5 |
95.73 |
488.71 |
|
F6 |
92.40 |
199.16 |
|
F7 |
91.18 |
446.84 |
|
F8 |
98.85 |
483.12 |
|
F9 |
94.18 |
412.36 |
|
|
|
|
F1 |
F2 |
|
|
|
|
F3 |
F4 |
|
|
|
|
F5 |
F6 |
|
|
|
|
F7 |
F8 |
|
|
|
|
F9 |
|
|
Figure 1. Optical Microscopic Images of Factorial Batches |
|
Vesicle size is an important parameter for the transdermal delivery of the drugs. Nanophox zetasizer was used to calculate the vesicle size for factorial batches. All the batches showed the vesicle size in nanometer range. The vesicle size of ethosomal suspension ranges from 199.16 to 484.46 nm.
Evaluation of ethosomal gel
Physical appearance
All the prepared ethosomal gel visually evaluated for their color and they were found to be smooth, homogeneous and white in color.
pH, drug content, in-vitro drug release
All the formulations were analyzed for pH, % drug content and % cumulative drug release. The results are shown in Table 2.
Table 2. pH, Drug Content and In-vitro Drug Release of Gel
|
Formulation code |
pH |
% Drug content |
% cumulative Drug release at 8h |
|
F1 |
6.6 |
90.29 |
72.10 |
|
F2 |
6.8 |
89.01 |
66.26 |
|
F3 |
6.9 |
90.23 |
62.92 |
|
F4 |
6.7 |
92.69 |
65.07 |
|
F5 |
7.0 |
91.25 |
67.05 |
|
F6 |
6.8 |
94.43 |
73.24 |
|
F7 |
6.6 |
88.16 |
68.18 |
|
F8 |
6.4 |
90.01 |
59.58 |
|
F9 |
6.7 |
80.54 |
70.11 |
Statistical analysis of factorial batches
Regression analysis was done to understand the effect of independent variables that is concentration of soya lecithin and alcohol on entrapment efficiency and percent drug release of repaglinide from factorial formulations. Nine batches were run through the Stat-Ease software. The best fit model for both the responses Y1 and Y2 was found to be linear model based on the p value which was less than 0.05. The p value was less than 0.0007 for % EE and less than 0.0003 for percent cumulative drug release.
The percent entrapment efficiency (% EE) of repaglinide in ethosomes ranged from 87.53 to 98.85%. The polynomial equation for the response was found to be shown in equation 3:
|
|
(3) |
As seen from the equation and observed experimentally, it can be concluded that the % EE of drug in ethosomes depends on the concentration of soya lecithin in the formulation. As the amount of soya lecithin increases in the formulation, the % EE of drug enhances. Whereas, ethanol has negative influence on % EE, as the concnetration of ethanol increases in the formulation the % EE decreased which can be observed from negative coefficient in the equation. The effect of soya lecithin on % EE can be seen as surface response curve in Figure 2.
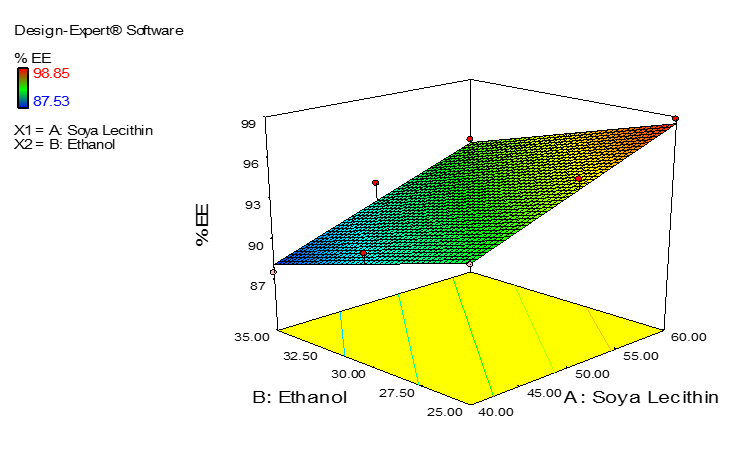 |
|
Figure 2. The Surface Response Curve Showing Effect of Soya Lecithin and Ethanol on the % EE of Repaglinide |
The percent cummulative drug release at 8 hour ranged from 59.58 to 73.25% and the polynomial equation for the response Y2 obatained from software was:
|
|
(4) |
The equation 4 indicates that concentration of ethanol plays a positive role in the release. As the conentration od ethanol increases in ethosomal formulations the drug release enhances. The effect can be seen in Figure 3.
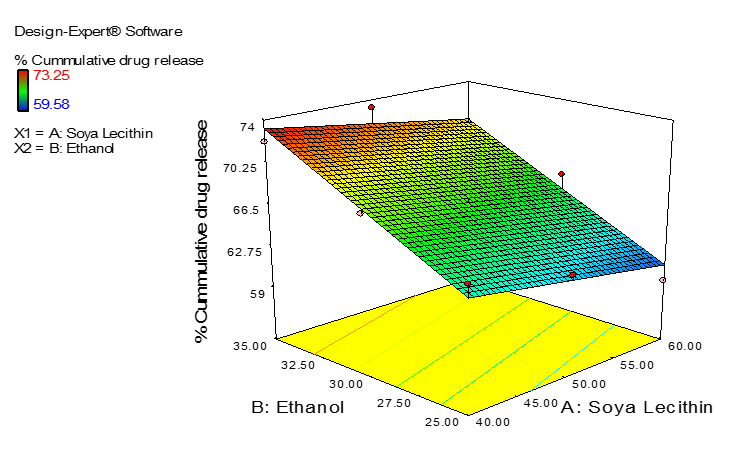 |
|
Figure 3. The Surface Response Curve Showing Effect of Soya Lecithin and Ethanol on Cumulative Percent Drug Release |
Based on the surface response curve and contour plot, optimization was done for factors by selecting the responses in the predetermined range and an overlay plot (Figure 4) was obtained. The optimized batch was prepared as per the overlay plot and evaluated. The concentration of soya lecithin and ethanol in optimized formulation was 55.19 mg and 34.61%. The ethosomal suspension was prepared and incorporated in gel. The entrapment efficiency of optimized ethosomal suspension was found to be 91.22%. The optimized ethosomal gel was smooth, homogeneous and creamy white in appearance. The pH of the gel was found to be 6.9 and the drug content was 90.45%. The cumulative percent drug release of repaglinide at 8h from gel was 69.81%. All the values obtained for % EE and percent drug release was nearer to that predicted by the software, indicating successful formulation of optimized batch.
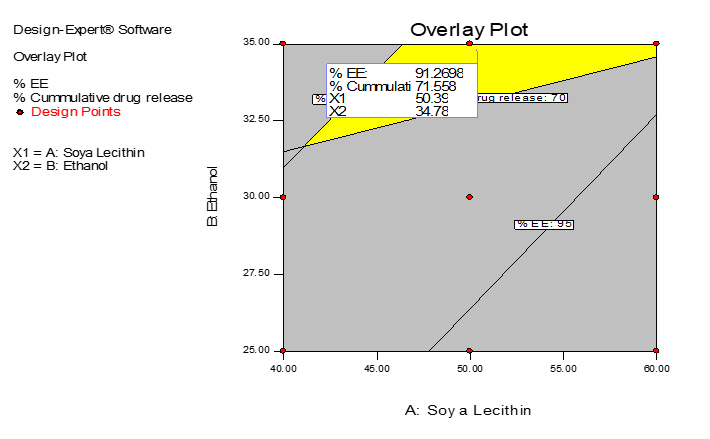 |
|
Figure 4. Overlay Plot of Region of Optimized Formulation |
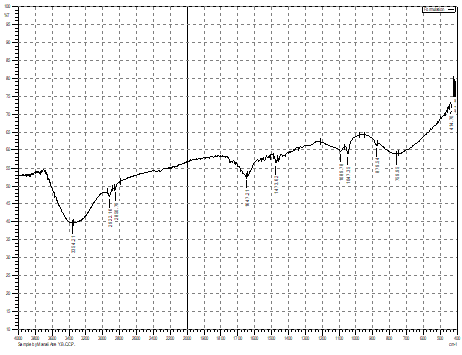
Characteristic peaks of optimized ethosomal formulation are shown in Figure 5a. The functional groups present such as N-H stretch, C-H Stretch, O-, C=O shows the bands in spectra which was comparable with FTIR spectra of pure repaglinide.
The SEM (Figure 5b) of optimized ethosomal formulation shows the spherical morphology of the vesicles. The vesicle size was found 198.92 nm and zeta potential was -13.42 mV.
 |
|
a) |
|
|
|
b) |
|
Figure 5. a) FTIR Spectra and b) SEM Analysis of Optimized Ethosomal Formulation |
CONCLUSION
The present study was done to formulate sustained release ethosomal gel of repaglinide as transdermal drug delivery system. The drug can be deleivered through the skin for treatment of diabetius mellitus type 2. The formulation can be successfully developed using design of experiment and can be used as one of the alternative to sustained the release of repaglanide through ethosomal gel preparation.
Acknowledgments: Authors are thankful to Y. B. Chavan College of Pharmacy, Aurangabad for providing us the facility to carry out the research work.
Conflict of interest: None
Financial support: None
Ethics statement: None