



|
Epidemiology of the Urinary Stones: A 6-Year Retrospective Study at Dezful-Iran Leila Kalani1, Neda Rashidi2, Shahzad Mehranfard3, Hadi Bahrami1, Narges Majidipour4, Ali Sadeghi Moghaddam5, Leila Masoudiyekta6* |
|
1MSc in Critical Care Nursing, School of Paramedical Sciences, Dezful University of medical sciences, Dezful, Iran. 2M.Sc In Operating Room, Department Operating Room, School of Paramedical Sciences, Dezful University of Medical Sciences, Dezful, Iran. 3MSc in Critical Care Nursing, School of Nursing and Midwifery, Dezful University of Medical Sciences, Dezful, Iran. 4Instructor of Neonatal Intensive Care Nursing, Department of Nursing, School of Nursing, Dezful University of Medical Sciences, Dezful, Iran. 5MSc in Medical-surgical Nursing, School of Nursing and Midwifery, Dezful University of medical sciences, Dezful, Iran. 6MSc in community health nursing, school of nursing and midwifery, Dezful University of Medical Sciences, Dezful, Iran. |
ABSTRACT
Introduction: The prevalence of urinary stones in different parts of the world is estimated to be in the range of 1-15% and Iran has identified as one of the "stone belt" countries with a prevalence of 2-3%. This study aimed to determine the epidemiology of urinary stones in patients referred to Ganjavian hospital at Dezful-Iran from 2010 to 2015. Materials and Methods: The study population included 519 adult patients over 15 years old with a diagnosis of urinary stone who were retrospectively assigned to the study. Data collection tools included a demographic checklist and patient biochemical tests. Results: The frequency of male and female patients was 69.6 and 30.4%, respectively. The mean age was 47.1 years. The patients’ medical history included urinary stone (43%), diabetes (18%), hypertension (29.7%), cardiac chronic disease (0.6%), hyperlipidemia (0.8%), chronic kidney disease (1%), prostatic hypertrophy (0.6%), urethral stricture (0.6%), urinary tract infection (0.6%), cyst (1.2%) and others were without underlying disease. Conclusion: Various epidemiological factors such as age, sex, hypertension, high blood sugar and history of medications are effective in getting urinary stone. So, by identifying the susceptible patients and teaching them, the burden of the disease on society and the individual can be reduced.
Key Words: Urinary stone, Patient, epidemiology, Dezful.
INTRODUCTION
Urinary stone is one of the worldwide diseases caused by the deposition of crystals made of oxalate-calcium, phosphate calcium or uric acid in the urinary tract [1]. The prevalence of kidney stones in different parts of the world is estimated to be in the range of 1% to 15% and Iran has been identified as one of the "stone belt" countries with a prevalence of 2% to 3% [2]. Various epidemiological and demographic factors such as age, sex, ethnicity, history of previous illnesses, social class, occupation, family history, nutritional factors, education level, income status [3] hypercalciuria, hyperoxaluria, hyperuricosuria, and hypercitraturia have been identified as the predisposing factors for urinary stones [4].
Today, the morbidity of urinary stones is reduced, but its long-term complications such as the risk of recurrence of stone formation, kidney failure, and uremia can be a burden for the patients in the future [5]. Moreover, the urinary stone disease is among the painful and costly disease. Hospitalization, medical interventions and the loss of working days impose a huge economic burden on the patient. Thus controlling this situation, particularly in developing countries, which relies heavily on preventing the formation of new stones and preventing the growth of stones that were previously there, is of great importance [6]. A study by Shirazi et al. showed that identifying and educating the patients with susceptible and at-risk kidney stones, the burden of the disease can be significantly reduced on the individual and society [7]. Pieces of literature have emphasized that more studies are needed to determine the conditions and factors that increase the risk of urinary stones in a certain population. Given the contradictory results of the previous pieces of literature on the urinary stones and its related factors, this study aimed to evaluate the epidemiology of urinary stones in patients referred to as the largest teaching hospital of Dezful-Iran namely Ganjavian hospital. To our knowledge, this is the first study in this region.
MATERIAL AND METHODS
This study was a retrospective and cross-sectional study to determine the epidemiology of urinary stones in patients referred to our hospital from January 2010 to December 2015. According to the study by Safarinejad [8] and
n=Z1-α/22.P(1-P)d2 formula, the sample size was estimated to be 519 individual patients. After primary coordination and approval of the university ethic committee, the researchers went to the medical records unit of the hospital and retrospectively investigate the records of patients with a definitive diagnosis of urinary stones. Each case was assigned a number from the total number of cases and according to the random number table; the samples were included in the study. The demographic data and biochemical tests of each patient were recorded. Inclusion criteria were patients over 15 years of age who given a definitive diagnosis of urinary stone disease. Records without data were excluded as incomplete.
From the patient profile, tests, and description of the operation, the following information was extracted: sex, diabetes, hypertension, urinary tract history, type of blood group, location of the stone, type of stone withdrawal procedure, Antihypertensive drugs, aspirin, and anti-diabetic use was included as well as quantitative variables including age, triglyceride levels, cholesterol levels, hypertension, and blood glucose levels. Statistical analysis was performed after transferring to the SPSS table. For descriptive statistics, mean, standard deviation and frequency distribution were used. SPSS software version 11.5 was used for statistical analysis.
RESULTS
The mean of patients’ age, systolic and diastolic blood pressure, triglyceride, cholesterol and fasting blood sugar is summarized in Table 1. The distribution of the patients according to literacy level is shown in Figure 1. The most common disease in the patients was found to be hypertension and diabetes with a frequency of 29.7% and 18%, respectively (Figure 2). Concerning to medical history of the patients, 80.3% had a history of diabetes, 43.5% a history of anti-hypertension and 6.6% a history of aspirin use. Most of the stones were in the ureter space (Table 2). The therapy action to remove the stones were TUL (transurethral lithotripsy) (63.2%), drug therapy (11.4%), cystolitholapexy (9.4%), ureteroscopy (6.4%), nephrolithotomy (5.4%), extracorporeal shock wave lithotripsy (3.9%) and nephrectomy (0.4%). 35% of patients had blood group A, 35% group O, 23.8% group B and 6.3% group AB (figure 3). 92.3% had positive RH and 7.7% had negative RH (figure 4). 40.9% of renal stones were in the right kidney and ureter, 36% in left kidney and ureter, 10.9% in bilateral and 12.2% in the bladder. 55.9% had triglyceride lower than 150 (normal) mg / dl, 14.7% triglyceride 150-199 (high) mg/dl, 26.5% triglyceride 200-499 (hyper) mg/dl and 2.9% had triglycerides greater than 499 (very high) mg/dL. 63% cholesterol lower than 200 mg/dL, 25.9% cholesterol 239-200 mg/dL (borderline) and 11.1% had high cholesterol levels of 239 mg/dL. 8.5% of patients had a history of antihyperlipidemic agents and 91.5% had no history of antihypertensive drugs.
DISCUSSION
Several factors are involved in the formation of urinary stones which are discussed in our study.
The mean age of the subjects in this study was 47.1 year and the highest incidence of kidney stones was in the 4th decade of life, which is consistent with Muhbes et al [9]. Inconsistent with our study, Strob et al showed that hospitalization rates for female patients were higher than male patients (52% vs. 22%), which could be due to fluid intake, obesity, and dietary changes.
In our study, the prevalence of kidney stones in male patients was higher than female patients that may be due to males exposed to hot Khuzestan climate, family history, insufficient fluid intake, inactivity in males and dietary habits.
A study by Jaber Muhbes [9] showed that the number of male patients was more than female patients and this study is consistent with our findings. This difference may be due to anatomical differences in the urinary tract of males and females. Due to the long urethra in men, it causes stagnation and accumulation of urine in the bladder and the formation of stones.
In our study, 33.9% of the patients had primary education. This result is consistent with the Muhbes et al [9] and can be concluded that people with low literacy levels may be exposed to kidney stones due to their lack of knowledge about dietary patterns and stone-forming factors.
Our study showed that 35% of the patients had an A and O blood group. However, in the study of Rafiq Anwar and Basha [10], the incidence of kidney stones was 37%, 36%, 19% and 8% for the O, B, A, and AB blood groups, respectively. A study by Rozanski et al [11] showed that the highest rate of morbidity kidney stones is present in women with A and O blood groups in the 4th decade of life while patients with blood group B had the highest morbidity kidney stone in the 5th decade of life. In our study, the prevalence of kidney stones in patients with O and A blood groups was higher than other blood groups, so this study is consistent with our study. In the Rozanski study, there was no significant relationship between kidney stone and blood group B, whereas in our study the frequency of patients with kidney stones with blood group B was 23.8%, which is inconsistent with our study. Given the limited number of studies, cultures, and geographical regions that are related to the incidence of kidney stones and blood groups. It is recommended that this study be conducted in different populations with a high sample size. Are blood groups effective on kidney stones?
In the Jaber Muhbes study [9], there was a relationship between kidney stone and aspirin use and 35% of patients had a history of aspirin intake and in our study, patients with a history of aspirin intake were 6.6%, which is consistent with the findings of our study. A study by Shang et al [12] showed that nephrolithiasis was associated with an increased risk of hypertension and suggested that a future high-quality randomized controlled trial be conducted to clarify the relationship between nephrolithiasis and hypertension. Given that 29.7% of patients in our study had hypertension and 43.5% of patients had a history of anti-hypertension medication, our study is consistent with the Shang study. The study by Kittanamongkolchai et al [13] showed that the severity, type, and treatment of kidney stones were not associated with hypertension, which is not consistent with our study.
A study by Kang et al [14] showed that patients with kidney stones with hypertriglyceridemia had higher urinary excretion of calcium, sodium, uric acid, magnesium, and potassium. In the study, people with high cholesterol with low-density lipoprotein had higher levels of sodium, magnesium, and potassium in the urine, while those with high cholesterol with low-density lipoprotein had lower sodium excretion. Hypertriglyceridemia is independently associated with recurrence of stone. The results of the study showed that serum lipid is associated with metabolic changes in the urine. Most importantly, hypertriglyceridemia is independently associated with an increased risk of stone recurrence in patients with a kidney stone. This study is consistent with our study. These results could be used to assist patients with dyslipidemia in preventing stone formation and recurrence. Weight control and dietary interventions can be one of the preventive methods against recurrent stone formation in patients with dyslipidemia.
The study by Chung et al. [1] showed that patients with kidney stones were at increased risk of diabetes after 5 years of follow-up. The data of the Chung study confirm further research on the role of insulin resistance in kidney stone pathogenesis and underlying mechanisms. Patients with urinary tract infections may require appropriate lifestyle changes and rapid monitoring of blood sugar monitoring.
Weinberg et al.'s [15] study showed that the severity of Type 2 diabetes, as measured by glucose control and homeostasis model assessment of insulin resistance, is an important risk factor for kidney stone disease and showed that patients with hemoglobin were more than 6.5% at risk for kidney stone.
The homeostasis model assessment of insulin resistance measures insulin sensitivity in patients with Type 2 diabetes, which are among the valid parameters and suggested that further studies are needed to confirm this relationship. Hyperglycemia and glycosuria caused by diabetes are involved in the renal transport of calcium, phosphorus and uric acid. Studies showed that patients with Type 2 diabetes have increased urinary calcium and phosphorus excretion. A study by Kabeya et al [16] showed that there was no relationship between insulin resistance parameters and kidney stone risk, which is inconsistent with our study.
A history of kidney stone is associated with an increased risk of chronic kidney disease; it is hypothesized that the cumulative effects of transient renal obstruction at the time of stone passage, renal mineral deposition, and kidney side injury (extracorporeal shock wave lithotripsy; laser ureteroscopy and lithotripsy) may cause nephrons and kidney function [17]. According to the study of Meschi et al. [18], there are ten rules to prevent the risk of kidney stone, including:
Suggestion:
It is recommended that further studies be conducted in a cohort with a high sample size to establish the relationship between kidney stones and related factors.
Limitations:
Patient records, diet, lifestyle, occupation, type of medication used, stone type analysis, BMI and biochemical tests had not been fully listed.
CONCLUSION:
Various epidemiological factors such as age, sex, hypertension, high blood sugar and history of medications are effective in getting urinary stone. So, by identifying the susceptible patients and teaching them, the burden of the disease on society and the individual can be reduced.
ACKNOWLEDGEMENT
This article was derived from a Research project with ethics code IR.DUMS.REC.1396.13 in Dezful University of Medical Sciences,
Financial support and sponsorship
Deputy of research and technology of Dezful University of Medical Sciences
Conflicts of interest
There are no conflicts of interest.
REFERENCES
Table 1: The mean and standard deviation of the age, systolic and diastolic blood pressure, triglyceride, cholesterol and fasting blood sugar according to patients' sex
|
Patients sex |
Number |
Age |
Systolic Blood Pressure |
Diastolic Blood Pressure |
Triglyceride |
Cholesterol |
Fasting Blood Sugar |
|
Males |
361 |
47±17 |
129±21 |
77±11 |
183±138 |
176±55 |
108±41 |
|
Females |
158 |
45±18 |
128±19 |
74±12 |
184±136 |
177±54 |
107±40 |
Table 2: Frequency Distribution of stone space
|
Space |
Frequency |
Percent |
|
kidney |
89 |
17.1 |
|
ureter |
368 |
70.9 |
|
bladder |
62 |
11.9 |
|
Total |
519 |
100.0 |
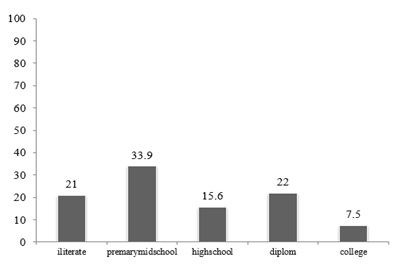
Figure 1: Frequency Distribution of the patients according to literacy level
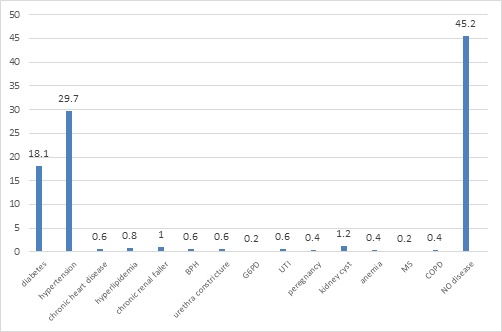
Figure 2: frequency Distribution of the comorbid' diseases
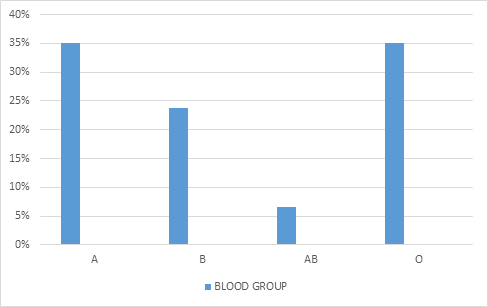
figure 3: frequency distribution analysis of ABO in renal calculi patients
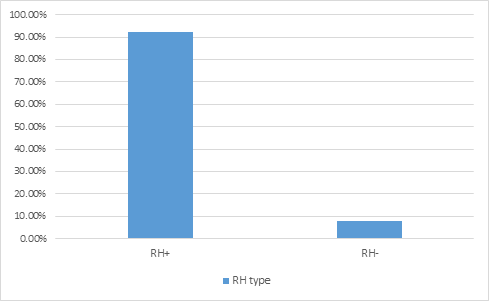
Figure 4: frequency distribution analysis of RH in renal calculi patients