



|
Effects of L-PRF and Advanced PRF Plus with Hydroxyapatite/Beta Tricalcium Phosphate in Bone Regeneration-A Histologic and Histomorphometric Study in Rabbits |
|
Ahmad Mogharehabed 1, Faegheh Fazeli 2, Zohreh Afshari 3, Jaber Yaghini 4* |
|
|
|
1Dental Implants Research Center, Department of Periodontics, Dental Research Institute, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran. 2Department of Periodontics, School of Dentistry, Zanjan University of Medical Science, Zanjan, Iran. 3Post Graduate Student, Dental Student's Research Committee, Department of Periodontics, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran. 4Dental Implants Research Center, Department of Periodontics, Dental Research Institute, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran. |
ABSTRACT
Introduction: So far, various methods have been used to accelerate the bone healing process. Among these methods is platelet-rich plasma (PRP) or platelet concentrate (PC), which provides high concentrations of growth factors. The aim of the present study was to compare the effect of Leucocyte and platelet-rich fibrin (L-PRF) and advance platelet-rich fibrin plus (APRF+) with biphasic calcium phosphate HA/βTCP (BCP) on bone regeneration by histological and histomorphometric investigation in Calvaria of rabbits. Materials and Methods: In the parietal bone of 12 rabbits, 4 bony defects were created and divided into 4 groups. 1) Control that was left blank, 2) HA/βTCP, 3) HA/βTCP + L-PRF, and 4) HA/βTCP + APRF Plus. Rabbits were randomly divided into 2 groups so that 6 rabbits were sacrificed at week 4 and the rest at week 8. The prepared samples were examined microscopically and histomorphometrically. Findings: In the group filled with BCP along with LPRF in the first and second months, the amount of newly formed bone was significantly higher than the BCP group alone. In concurrent use with APRF+, the amount of new bone formation was greater compared to other groups in the first month, and the cavity was filled with mature bone in the second month. Overall osteoblast, osteocyte, and osteon amounts were specifically higher in BCP+LPRF and BCP+APRF plus groups than other groups. In addition, all three treatment groups showed a significantly higher amount of osteon than the control group in the second month. Conclusion: BCP alloplast substance in combination with LPRF or APRF+ shows a higher amount of ossification compared to the use of bone substitute substance alone.
Key Words: Bone Regeneration, L-PRF, APRF+, histomorphometry, Rabbit.
Bone reconstruction and regeneration in bone lesions are considered a predictable and accepted method in periodontal, implant, and ridge reconstruction treatments [1]. Different methods and various graft materials including autograft, allograft, alloplast, and xenograft have been used for this purpose [2-4]. Intraoral and extraoral autogenous bone grafts have been considered as the gold standard in bone reconstruction and regeneration. Although this technique has high efficiency, it requires another surgery that is associated with higher morbidity. Many surgeons prefer autografts because there is no possibility of transplant rejection [5]. However, the existence of conditions and problems such as performing two concurrent surgeries, the limited size of the graft, increased likelihood of bleeding occurrence, and so on, have caused specialists to pay attention to the appropriate alternative to autografts, namely allografts as well [6].
Frozen and demineralized allografts such as DFDBA (demineralized freeze-dried bone graft) and frozen FDBA (freeze-dried bone graft) are commonly used by clinicians to reconstruct alveolar ridge [7]. On the other hand, calcium-phosphate-based alloplastics appear to be a proper alternative to autografts and allografts [8]. Hydroxyapatite is the major inorganic component of the bone matrix, and synthetic hydroxyapatite when used as a bone graft, shows high degrees of biocompatibility, provides proper support for cellular activity, stimulates ossification through osteoconduction, and is usually gently replaced by host bone [9]. Tricalcium phosphate is also an absorbable bioceramic that its absorption rate is faster than bone formation. Another type of alloplasts is biphasic calcium phosphate (BCP), which is actually a combination of hydroxyapatite with the slow-absorption capability and tricalcium phosphate with fast-absorption capability, which is preferred as an osteoconductive matrix to maintain space in bone regeneration [10].
Today, methods to affect the bone healing process have been investigated and researched. Among these methods include platelet-rich plasma (PRP) or platelet concentrate (PC), which provides high concentrations of growth factors that have a direct effect on osteoblasts, periodontal ligament, and epithelial cells [11]. The first generation of platelet derivatives, which included platelet-rich plasma and growth factor-rich plasma, emerged [12]. After PRP, various generations of platelet-rich fibrin (PRF) were introduced. PRF was first introduced in France in 2001 by Choukroun et al. [13]. Leukocyte-and Platelet Rich Fibrin (L-PRF) belongs to the second generation of platelet derivatives [13]. L-PRF contains a dense fibrin network in which platelets and leukocytes have been trapped [14]. Based on the results obtained from a systematic investigation, it can be concluded that despite the lack of strong evidence, L-PRF can have positive effects on bone regeneration and osteointegration [15]. In the next generation of platelet-rich fibrins, less centrifugation speed and less force were used, resulting in the formation of advanced PRF, whose evident characteristic is a porous structure and a higher number of leukocytes, which causes more cell infiltration, vascularization, and release of growth factors [16]. In the next generation of PRFs, with a slight reduction in centrifuge time compared to advanced PRF and maintaining the force similar to it, advanced PRF plus (APRF+) emerged, which releases more growth factors than the previous generation [16].
A study in 2016 by Yen-Lan Chang et al. showed that the HLA/HA-βTCP combination had a significant effect on the bone reconstruction of Calvaria of rabbits [9, 17]. The result of an animal study showed that the combination of PRF with HA+βTCP significantly increased bone formation at weeks 4 and 8 compared with using PRF and βTCP alone [18]. Histological and serological investigations in another study showed more new bone formation in the combination of PRF and βTCP than the use of each one alone [19]. However, in a study in 2015 Knapen et al. stated that L-PRF did not appear to have an additional effect on bone quality and quantity [20].
Based on the results obtained from a systematic investigation in 2016 conducted by Castro et al. on the regenerative potential of L-PRF, it can be concluded that despite the shortage of strong evidence, L-PRF can have a positive effect on bone regeneration and osteointegration. It was also shown that faster bone healing was observed in sinus floor elevation, both in the lateral window method and in the trans alveolar method, when L-PRF was used along with xenografts [11]. Also, the results obtained from the systematic review of Miron et al. in 2017 showed that most researches support the use of PRF in the bone regeneration of periodontal hard tissue. It was also concluded that PRF improves soft tissue generation and reduces dimensional changes after tooth extraction [21].
Investigating the effect of L-PRF on bone regeneration in the field of dentistry has increased in recent years. However, since the effect of L-PRF on bone regeneration is contradictory and also studies conducted regarding the effect of various generations of PRF on bone regeneration in vivo and on animals are limited, performing the present study seems to be necessary. The aim of the present study was to compare the effect of L-PRF and APRF plus with biphasic calcium phosphate HA/βTCP on bone regeneration by histological and histomorphometric investigation in Calvaria of rabbits.
MATERIALS AND METHODS:
Animals:
Twelve male New Zealand white rabbits with an average age of 6 months and an average weight of 3 to 3.5 kg were used in this study. All stages and methods of doing this study were confirmed by the Ethics Committee of Animal Studies of Isfahan University of Medical Sciences and approved with the code of IR.MUI.RESEARCH.REC.1398.115.
Study design:
The synthetic substance HA/βTCP was prepared from Nova Company, that in this type HA and βTCP were combined in a ratio of 60 to 40. Also, for the preparation of LPRF and APRF+, special interlock centrifuge devices were used.
In order to randomly divide the rabbits, they were coded from numbers 1 to 12, then the numbers were selected randomly and blinded, and a group of 6 was selected for investigating at week 4 and the other group was selected for investigating at week 8. It was also done randomly to fill 4 defects in the rabbits' heads.
Surgical Procedure:
The environment and surfaces in which the surgery is performed are disinfected with a biological surface disinfectant solution (Sarfoscept Scrub). To anesthetize rabbit, Ketamine hydrochloride 10% (Alfasan, Woerden, Netherlands) at a dose of 30 mg⁄kg and Xylazine 2% (Alfasan, Woerden, Netherlands) at a dose of 3 mg⁄kg intramuscularly in the upper outer 1.4 of the thigh quadriceps muscle of the animal is injected. The incision site on the animal's head is scrubbed with betadine, then the hairs in the desired area are carefully and atheromatically shaved to prevent the emergence of wound and infection in this area. Depending on the weight, each animal is premedicated by intramuscular injection of sulfamethoxazole, trimethoprim, and meloxicam.
For local anesthesia and bleeding control, lidocaine 2% was injected into the skin of the midline area of the cranium. With a surgical blade number 15, the surgical incision was made anterior-posteriorly, approximately 4 cm from the frontal bone to the occipital. The skin, periost, and muscles of the area were then carefully reflected by the periosteal elevator (Molt # 9) and the parietal and frontal bones were exposed. When using 8-mm terfain, injectable wash serum was used as external irrigation to avoid overheating. The bone fragments were gently removed. There were 4 bony defects created in the parietal bone of rabbits and the defects were divided into 4 groups: 1) control group (group A) in which the defect was not filled with any substance, 2) HA/βTCP group (group B) that the cavity was filled with biphasic calcium phosphate alone, 3) HA/βTCP + L-PRF group (group C) that the cavity was filled with a mixture of these two substances, and 4) HA/βTCP group along with A-PRF plus that the cavity was filled with a combination of these two substances (fig. 3).
LPRF and APRF+ Preparation:
Blood samples were taken from the rabbits directly from the heart and under general anesthesia. L-PRF was prepared according to the Choukroun protocol. Blood tubes were placed at the centrifuge speed of 400 g for 10 minutes. The L-PRF clot was isolated from red blood cells and was placed in combination with biphasic calcium phosphate in the lesion [22].
To prepare APRF+, about 10 ml of rabbit blood was placed in a tube for 8 minutes at the centrifuge speed of 208 g and 1300 rpm. The obtained product was placed in the cavity in combination with biphasic calcium phosphate.
After placing the material in the periost cavities, it was sutured with 4-0 Vickeryl suture and continuously sutured and the Calvaria skin was sutured continuously with 3-0 nylon suture. After surgery, each animal was given antibiotics 10% enrofloxacin intramuscularly at a dose of 5 mg/kg twice daily for one week and for analgesia 10% ketoprofen intramuscularly at a dose of 1 mg/kg once daily for one week. Finally, rabbits were considered in two groups and after a healing period of 4 weeks and 8 weeks, they were euthanized. The euthanasia of animals was performed by injecting intravenously with pentobarbital sodium 0.22 ml/kg for each sample. After 5 minutes and ensuring complete cessation of heart rate and respiration and observing mydriasis, the preparation of samples was done.
Samples Preparation:
To prepare the samples, we placed each of the samples separately in a 10% formalin container. The samples remained in the container for approximately 10 days for complete fixation. The skin and soft tissues were then completely removed and only bone fragments including the frontal, parietal, and upper rim bones of the orbital cavity were placed in the formic acid solution for 12 days for decalcification. At this interval, the samples were monitored daily for sufficient softening until they were fully prepared for incision by microtome device. At least four 6-micron incisions were prepared consecutively by a microtome device in the center around the largest diameter. The incisions were processed in a histokinette device for tissue processing for 24 hours, and they were molded with the aid of paraffin and prepared from the resulting micron incision block, and the samples were prepared by hematoxylin and eosin (H&E) and Mason Trichrome (MT) staining and slides were examined by a light microscope.
Histological and Histomorphometric Analysis:
Histological sections were examined by two independent pathologists with a light microscope (Olympus BX51; Olympus, Tokyo, Japan).
The amount of newly formed bone in the entire area of the defect cross-section was evaluated. To differentiate the control cavity from the new bone formation in the defect areas, areas with lacunae containing live osteocytes were considered as a newly formed bone. In addition, for histomorphometric analysis, the number of cells including fibroblasts, osteoblasts, and osteon using Image-Pro Plus® V.6 computer software (Media Cybernetics, Inc., Silver Spring, USA) were calculated and analyzed. A magnification of ×400 was used to count the cells and the calculation was repeated for 4 areas. Finally, the average number of each scale was recorded.
Statistical Analysis:
Data analysis was performed using SPSS 21 statistical software at a statistical significance level of 0.05. Descriptive statistics were reported using mean, standard deviation, frequency, and percentage. According to the scale of variables, a one-way ANOVA test was used to examine the dependent variables of the study in the groups under investigation (if the presuppositions of this test are established). In case of the significance of the analysis of variance, Post hoc tests such as Tukey were also used to investigate the relationship of the pair between groups. Also, if the normality hypothesis is not established, non-parametric tests equivalent to Kruskal-Wallis were used and the Mann-Whitney test was used to investigate the pairs of groups by applying a significant level according to the number of tests. Also, in order to determine the effect of methods under investigation in the fourth and eighth weeks, the independent t-test or its equivalent, the Mann-Whitney test, was used in non-parametric conditions.
RESULTS:
All animals recovered from surgery without complications and gained weight normally without signs of pain. The samples were allocated based on which the investigated variables in four control groups: BCP, BCP/LPRF (BCPL), and BCP/APRF+ (BCPA) were determined to investigate the objectives of the study.
The results of the analysis in the first and second-month samples for the mean number of fibroblasts, osteoblasts, number of osteons, percentage of bone formation, percentage of fibrous tissue in the groups of control, BCP, BCPL, and BCPA have been mentioned in Table 1.
Table 1: mean number of fibroblasts, osteoblasts, osteons, bone formation and fibrous tissue results in each group
|
First month (Mean+SD) |
Control |
BCP |
BCPL |
BCPA |
|
Fibroblast abundance distribution |
186.15+3.8 |
55.8+5.8 |
54.8+0.7 |
52.8+5.4 |
|
Osteoblast abundance distribution |
3.2+7.4 |
5.3+7.3 |
20.3+2.5 |
41.8+8.0 |
|
Osteon abundance distribution |
0.0+0.0 |
0.0+7.5 |
1.1+7.03 |
5.1+8.8 |
|
Osseous tissue formation percentage |
1.15+0.8 |
2.8+5.8 |
4.8+2.7 |
13.8+2.4 |
|
Fibrous tissue formation percentage |
83.5+3.5 |
34.5+5.2 |
24.5+3.6 |
32.8+8.4 |
|
Second month (Mean+SD) |
|
|
|
|
|
Fibroblast abundance distribution |
105.14+5.9 |
75.8+5.5 |
46.8+2.3 |
18.4+3.7 |
|
Osteoblast abundance distribution |
11.4+0.6 |
57.13+0.9 |
61.8+7.6 |
86.9+8.2 |
|
Osteon abundance distribution |
0.0+5.8 |
3.1+8.3 |
11.3+2.3 |
23.5+3.8 |
|
Osseous tissue formation percentage |
5.2+3.1 |
16.5+8.2 |
36.5+3.8 |
73.5+7.7 |
|
Fibrous tissue formation percentage |
77.9+3.9 |
58.7+7.6 |
49.8+0.9 |
23.4+8.9 |
There was a statistically significant difference in the two rabbit samples of the first and second month in terms of fibroblast cell number in the groups of control, BCP and BCPA, while this difference was not significant in the BCPL group. A statistically significant difference was observed in all groups regarding the number of osteoblasts and the percentage of ossification. Also, regarding the number of osteons and the percentage of fibrous tissue, the difference was significant in the three treatment groups, while this difference was not significant in the control group.
In the analysis performed between the studied groups both in the first month and in the second-month samples in terms of the number of fibroblast cell, osteoblast, osteon number, ossification, percentage of fibrous tissue formation between the 4 groups, this difference was statistically significant.
In the first-month samples, the frequency of fibroblasts was highest and lowest in the control and BCPA groups, respectively. The number of osteoblast cells was the lowest and highest in the control and BCPA groups, respectively. Comparison of dispersion and the mean number of osteon in the first-month samples showed that the number of these cells was zero in the control group and the highest in the BCPA group. The mean percentage of bone and tissue fibrous formation in the first-month sample was the lowest and highest in the control and BCPA groups, respectively (Fig. 1).
Comparison of dispersion and the mean number of fibroblasts in the samples of the second month showed that the frequency of fibroblast cells was the highest and lowest in the control and BCPA group. Also, the number of osteoblasts is the lowest in the control group and is the highest in the BCPL group. Comparison of dispersion and the mean number of osteon cells and the percentage of new bone formation in second-month sample rabbits shows that the number of these cells is the lowest in the control group, and is the highest in the BCPA groups, respectively. Comparison of dispersion and the mean percentage of fibrous tissue formation in the second-month rabbit sample showed that this variable was the highest in the control group, and lowest in the BCPA group (Fig. 2).
In general, the results of statistical analysis showed that a statistically significant difference was observed between the variables of osteoblast and osteon number and the percentage of new bone formation in the first month and the second-month samples. While the mean percentage of fibrous tissue formation did not have a significant difference between the first and second-month samples.
DISCUSSION:
The effect of L-PRF on osteoblasts to improve bone regeneration has been studied and promising results have been shown [23-25]. However, the clinical benefits of various derivatives of this substance in bone regeneration are still unknown. In 2012, Kim et al. used a combination of TCP and L-PRF to reconstruct bone defects in calvaria of rabbits and showed the formation of large amounts of new bone 2, 4, 6, and 8 weeks after surgery [26]. Gradually, with the advent of new derivatives of growth enhancer substances, attention was drawn to these substances. In this study, we decided to investigate the effect of L-PRF and APRF+ in a standard model along with BCP. The results showed that the overall amount of osteoblast, osteocyte, and osteon in the BCP/L-PRF and BCP/APRF+ groups were specifically higher than the other groups. These results support increased proliferation, differentiation, and protein production in the presence of L-PRF [23-25].
According to the findings of the present study, the amount of fibrous tissue produced after one month was almost similar in the BCPA, BCPL, and BCP groups, but it was significantly lower than the control group. This may be because although L-PRF can stimulate fibroblast migration and angiogenesis, and its fibrin structure can be perforated by fibroblasts [12, 27], BCP acts as a scaffold under soft tissue in treatment groups, and plays a role as an osteoconductive substance and as a space preserving agent, and allows the regeneration space to be provided. While in the control group, this factor was not present, so the proliferation of fibrous tissue in this group quickly occupies the space required for regeneration. However, the analyses of the second month showed that the amount of fibrous tissue in the BCP and BCPL groups showed a significant increase compared to the first-month, and in the other two groups, the decrease of these amounts was observed. However, the total amount of fibrous tissue in the BCPL and BCPA groups was lower than the other two groups.
In all variables of this study, the improvement in osteogenesis process and cells was in favor of bone formation as well as a decrease in the amount of fibroblasts and fibrous tissue formation in the BCPL and BCPA groups compared to the BCP and control groups; although some of these differences were not significant. This point confirms the effect of growth factors existing in these substances on bone regeneration.
Histopathological results show that in the group filled with biphasic calcium phosphate, after one month of a lesion with the new bone, the ossifier substance and fibrous tissue were filled, while after 2 months, this cavity was slightly filled with bone and mostly contained fibrous connective tissue. However, adding L-PRF or APRF+ significantly increased the osteogenesis ratio. These findings are consistent with the recent study of the group of Fahimipour et al. By adding L-PRF to a βTCP-reinforced collagen scaffold, they observed an increase in lamellar bone regeneration in a rabbit model [28]. Also, in a study by Ozdemir et al. in 2013 on New Zealand rabbit Calvaria, after one month, more new bone areas were observed in the PRF group than in the control group, and no statistically significant difference was observed between the PRF group and Bio-oss and BCP. Also, three months after healing, in a study by Ozdemir et al. in 2013 on PRF and Bio-oss, they showed higher bone formation than the control group, which is consistent with the results of the present study [29].
A study by Bolukbasl et al. in 2013 examined the histological effect of PRF in combination with BCP on bone regeneration. In this study, 5 mm defects were created on both tibias of 6 sheep. One defect was left empty and the rest were filled with PRF, BCP, and a combination of the two. Animals were sacrificed on days 10, 20, and 40. On day 40, the PRF + BCP combination showed the highest amount of new bone formation. Although the time periods under investigation and the animal under study were different from the present study, it ultimately showed the positive effect of PRF on bone regeneration [30].
L-PRF and APRF+ are both rich in leukocytes, so they can have an antibacterial effect. Although this point was not investigated in the present study and more studies are needed in this field, it seems that this factor can be effective in improving regeneration and wound healing because the presence of microbial agents can interfere with this process and faster clearing the surgical site from these factors can be effective in reducing the time required to begin the process of tissue reconstruction.
CONCLUSION
In bone regeneration treatments, one of the best treatment options is to use bone substitutes such as alloplast substances in combination with L-PRF or APRF+, which show a higher amount of ossification compared to using bone substitute substances alone.
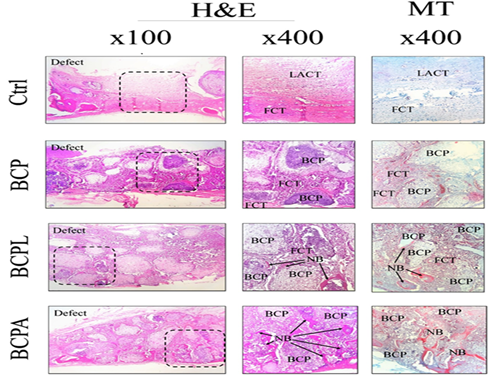
Figure 1. Histopathological findings of experimental calvarial defects in different groups at 1-month post-surgery. BCP: biphasic calcium phosphate, LACT: loose areolar connective tissue, FCT: fibrous connective tissue, NB: new bone formation, H&E and MT staining.
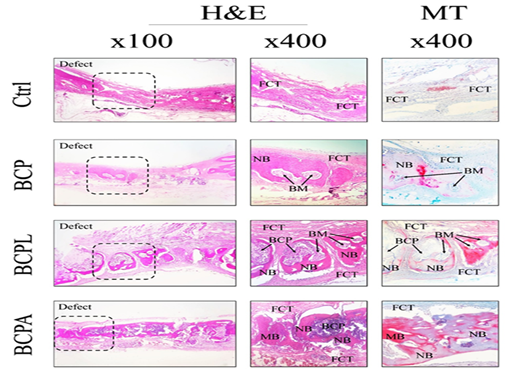
Figure 2. Histopathological findings of experimental calvarial defects in different groups at 2-month post-surgery. FCT: fibrous connective tissue, BCP: biphasic calcium phosphate, LACT: loose areolar connective tissue, FCT: fibrous connective tissue, NB: new bone formation, BM: bone marrow, MB: mature bone, H&E and MT staining.
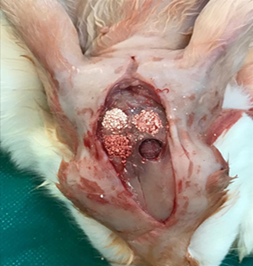
Figure 3: Preparing the case and control cavities and filling them with biomaterials.
Conflict of Interest and Sources of Funding Statement:
The authors declare that there are no conflicts of interest in this study
REFERENCES