




The present research examined the diuretic potential of the ethanolic derivatives of Bauhinia tomentosa Linn. (B. tomentosa) roots. The roots of this plant have been traditionally used for their diuretic effects, but scientific evidence supporting this claim is limited. The study utilized Wistar rats and evaluated urine volume, electrolyte excretion, and renal function to assess the diuretic functionality of the derivative. The findings demonstrated that the ethanolic extract considerably increased urine output and improved the excretion of Na, K, and Ch compared to the control group. The diuretic effect was dose-dependent, with higher doses producing a greater effect. Furthermore, the diuretic activity of the derivative was comparable to that of the standard diuretic drug, furosemide. Statistical analysis confirmed the significance of the findings (P < 0.001). The phytochemical composition of the extract, which includes flavonoids, alkaloids, tannins, saponins, and phenolic compounds, may contribute to its diuretic properties. The study suggests that B. tomentosa roots have potential as a natural diuretic agent, offering advantages over synthetic diuretics with fewer side effects. Further studies are needed to identify the active ingredient responsible for the observed diuretic effect and to elucidate the underlying mechanisms. The outcome is in line with the local use of B. tomentosa roots as a diuretic and warrants further investigation into their therapeutic applications.
INTRODUCTION
Various civilizations have recognized and used medicinal plants' healing potential for ages [1]. In recent years, there has been a greater emphasis on investigating the pharmacological effects of traditional plant medicines as a source of new medicinal molecules [2]. Bauhinia tomentosa Linn. (B. tomentosa), commonly referred to as “Yellow Bauhinia” is one such plant that has a long history of usage in numerous systems of medicine [3].
B. tomentosa belongs to the Fabaceae family and is indigenous to tropical and subtropical regions [4]. Different parts of this plant, including leaves, flowers, bark, and roots, have been traditionally employed for the management of several ailments, including diuretic effects [5]. Diuretics are substances that promote increased urine production, aiding in the elimination of excess fluids and waste products from the body. The putative diuretic properties of B. tomentosa roots have drawn considerable attention in conventional medical practices [6]. Although there is anecdotal support for its diuretic effects, scientific validation and knowledge of the underlying mechanisms are lacking. The roots of B. tomentosa contain a variety of bioactive substances, including flavonoids, alkaloids, tannins, saponins, and phenolic compounds, according to phytochemical studies [7]. These constituents are known to possess diverse pharmacological activities, including diuretic properties. Therefore, exploring the diuretic potential of the ethanolic extract of B. tomentosa roots becomes imperative for better understanding its medicinal value and therapeutic applications.
Treatment for ailments like hypertension, edema, renal dysfunction, and congestive heart failure often involves the use of diuretic drugs [8]. Currently, available synthetic diuretics have several negative side effects, including electrolyte imbalances and renal impairment [9]. There is consequently an increasing interest in finding safer and more potent diuretic substances from natural sources, such as medicinal herbs [10]. This study seeks to examine the diuretic capacity of the ethanolic extract made from B. tomentosa roots in light of the local use of these roots as a diuretic agent and the paucity of scientific data to support this claim. Utilizing experimental animal models, the study measures the characteristics of urine volume, electrolyte excretion, and renal function to determine diuretic activity [11]. The findings of this research may support the traditional usage of B. tomentosa roots as a diuretic and open the door to further investigation of their medicinal potential. Furthermore, the discovery of the causative agent of the observed diuretic effect may lead to the development of new, less dangerous diuretics.
MATERIALS AND METHODS
The roots of B. tomentosa were obtained from a local village in Mangalore and authenticated by botanist, Pilikula Nisargadhama, Vamanjoor, Mangalore. It's stored at the library of the dept for reference in the future. The current study was carried out at the Srinivas College of Pharmacy's Department of Pharmacology.
Plant extract
The roots of B. tomentosa were sun-dried and roughly pulverized. A weighed amount (200 g) of powder was then subjected to continuous hot extraction with ethanol in a Soxhlet apparatus. The extract was filtered through a cotton plug, followed by Whatman filter paper (No. 1), and dried at 40-50 °C to get a blackish-green semisolid material for final usage. The extract was stored in the refrigerator at 4–8 °C.
Animals
The following research used Wistar rats, which weigh between 150 and 200 g for both sexes. Five groups of animals, each with six rats, were used. The animals were kept in a consistent 12-hour cycle of light and darkness and provided regular pelleted food and purified water as needed. All animals were kept in lab settings for 5 days before the experiment [12]. Before the experiment, the animals were denied food and drink for 18 hours. The ethical guidelines were followed during the execution of each experiment.
Drugs and chemicals
Furosemide 20 mg/kg body weight (Sanofi India Limited), EEBT 100, 200, and 400 mg/kg body weight, and distilled water.
Experimental design
Acute toxicity studies
Following the guidelines provided by the Organization for Economic Cooperation and Development (OECD) on testing acute toxicity, an investigation was carried out. During the 24- to 72-hour observation periods, no adverse effects or death were seen in albino rats exposed to EEBT at doses up to 2 gm/kg, p.o. The rats were continuously examined for 5 hours throughout this time to check for any obvious behavioral, neurological, or autonomic toxic effects. After 24 to 72 hours, the animals were sacrificed [13].
Lipchitz method for diuretic activity
Wister rats were divided into 5 groups of n = 6 each.
Group I – Received normal saline (25 mL/kg) orally.
Group II – Received furosemide (20 mg/kg) orally.
Group III – received 100 mg/kg EEBT root orally.
Group IV – received 200 mg/kg EEBT root orally.
Group V – received 400 mg/kg EEBT root orally.
Group 1 served as control (vehicle treatment) and group II served as standard (20 mg furosemide per kg body weight). Group III, Group IV, and Group V were treated with ethanolic extract of B. tomentosa phosphorus root at 100 mg/kg, 200 mg/kg, and 400 mg/kg body weight (orally). Immediately after dosing, rat/rats in each cage were placed in metabolic cages specifically designed to separate wine and feces and maintained at room temperature of 25 ± 0.50 °C. The urine was gathered by using a cylinder for up to six hours. In the course of this, no food or water was made available to animals. Urine samples were stored in the refrigerator until Na+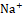 , K+
, K+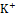 , and Cl-
, and Cl-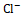 levels were calculated. The amount of urine collected for each group was quantified. No preservatives were added to the urine samples during storage. By utilizing 0.02N AgNO3 with 5% potassium chromate as an indicator, the amounts of urine Na+ and K+ were measured by flame photometry, and the quantity of Cl- was estimated titrimetrically.
levels were calculated. The amount of urine collected for each group was quantified. No preservatives were added to the urine samples during storage. By utilizing 0.02N AgNO3 with 5% potassium chromate as an indicator, the amounts of urine Na+ and K+ were measured by flame photometry, and the quantity of Cl- was estimated titrimetrically.
Statistical analysis
By using the outcome parameters' Mean values and Standard Deviation, the impacts of EEBT were estimated. The analysis of variance (ANOVA) method was used to compare the outcomes of the investigated medicines. ANOVA was used to analyze the data, and Dunnett's test was then performed. The significance level was set at P < 0.05. Graph-pad Prism software was used for all of the statistical analysis.
RESULTS AND DISCUSSION
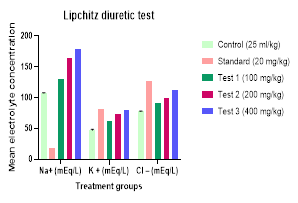
The findings from the assessment of the EEBT diuretic action were displayed in Table 1, Figures 1 and 2. According to the results, EEBT significantly increased urine output and increased salt, potassium, and chloride excretion when compared to control, indicating that it had a diuretic effect. It was found that the effect of EEBT was dose-dependent, meaning that among the three dosages examined, the higher dose generated a greater effect. The diuretic impact seen following treatment with EEBT was found to be considerable in terms of urine output, salt, potassium, and chloride concentrations when compared with the widely used diuretic medicine furosemide. Analyses of urine electrolyte concentrations showed that EEBT significantly raised the concentrations of the ions Na+ , K+
, K+ , and Cl-
, and Cl-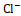 .
.
Table 1. Effects of oral administration of Ethanolic root extract of Mimosa pudica on urine volume and electrolyte excretion
|
Treatment |
Dose (Oral) |
Urine volume (ml) |
Na+ (mEq/L) |
K + (mEq/L) |
Cl – (mEq/L) |
|
Control |
25 ml/kg |
13.16 ± 0.59 |
107.35 ± 0.73 |
47.19 ± 1.54 |
77.58 ± 0.85 |
|
Standard |
20 mg/kg |
20.26 ± 0.03*** |
188.49 + 2.45*** |
81.64 ± 1.28*** |
127.41 ± 1.74*** |
|
Test- 1 |
100 mg/kg |
12.81 ± 0.29*** |
129.40 ± 2.80*** |
62.35 ± 0.06*** |
91.41 ±0.99*** |
|
Test- 2 |
200 mg/kg |
14.01 ± 0.38*** |
164.99± 2.00*** |
74.01 ± 2.77*** |
100.06 ± 2.25*** |
|
Test- 3 |
400 mg/kg |
16.13 ± 0.92*** |
179.17 ± 0.69*** |
80.23 ± 0.47*** |
112.96 ± 1.05*** |
N = 6, Values expressed as mean±SEM. ***Significance at P < 0.001. Compared with the control group (one-way ANOVA followed by Dunnett’s ‘t’-test). BW: Body weight, EEBT: Ethanolic extract of B. tomentosa root.
|
|
|
Figure 1. Comparative profile of volume of urine output after oral administration of 100, 200, and 400 mg/kg of EEBT. |
 |
|
Figure 2. Comparative profile of concentrations of the ions Na+ |
Fluid overload can lead to complications like renal failure and hypertension [14]. Diuretic therapy helps in managing these conditions by reducing fluid volume and relieving the strain on the kidneys and cardiovascular system [15]. The most common reason for hospitalization for those over 65 is heart failure. Worldwide, more than 20 million people suffer from heart failure [16]. According to estimates, India has a prevalence of 1.3 to 4.6 million cases of heart failure and an annual incidence of 0.5 to 1.8 million [17]. Despite high rates of morbidity and mortality, little is known about the pathophysiologic processes of heart failure or how to treat it [18]. Even with the introduction of more modern and selective drugs, their adverse effect profiles are a drawback, and only a small percentage of cases exhibit resistance to standard therapy [19].
Diuretic agents have a critical function in the management of conditions such as hypertension, edema, renal dysfunction, and congestive heart failure [20]. Synthetic diuretics currently available in the market, including furosemide, are associated with various adverse effects, such as electrolyte imbalances and renal dysfunction. Depending on the class of diuretic, many routes are involved in the drug's activity. For example, loop diuretics like furosemide act by inhibiting the Na+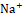 , K+
, K+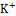 , and Cl-
, and Cl-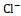 co-transporter in the thick ascending limb of the loop of Henle, leading to increased excretion of Na+
co-transporter in the thick ascending limb of the loop of Henle, leading to increased excretion of Na+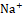 , K+
, K+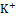 , and Cl-
, and Cl-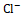 . This inhibitory effect disrupts the reabsorption of these ions, resulting in osmotic diuresis and subsequent water loss. Thiazide diuretics act on the distal convoluted tubule by inhibiting the Na+
. This inhibitory effect disrupts the reabsorption of these ions, resulting in osmotic diuresis and subsequent water loss. Thiazide diuretics act on the distal convoluted tubule by inhibiting the Na+ 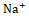 and Cl-
and Cl- 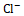 co-transporter, while potassium-sparing diuretics act on the collecting ducts to promote Na+
co-transporter, while potassium-sparing diuretics act on the collecting ducts to promote Na+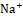 excretion while reducing K+
excretion while reducing K+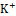 excretion [21]. Therefore, there is a need to explore safer and more effective diuretic agents from natural sources, such as medicinal plants.
excretion [21]. Therefore, there is a need to explore safer and more effective diuretic agents from natural sources, such as medicinal plants.
The traditional use of B. tomentosa roots as a diuretic agent is supported by the findings of this study. The increase in urine volume and electrolyte excretion indicates the potential of EEBT in promoting diuresis, which can aid in the elimination of excess fluid and waste products from the body. The exact mechanism of action of EEBT as a diuretic agent is not elucidated in this study. However, previous phytochemical investigations of B. tomentosa roots have revealed the presence of various bioactive compounds, including flavonoids, alkaloids, tannins, saponins, and phenolic compounds. These constituents have been reported to possess diverse pharmacological activities, including diuretic properties. The combined action of these bioactive compounds in EEBT may contribute to its diuretic effect. The use of EEBT as a diuretic agent offers potential advantages over synthetic diuretics. Natural products derived from medicinal plants often exhibit a broad spectrum of pharmacological activities with fewer side effects compared to synthetic drugs. By validating the traditional use of B. tomentosa roots and identifying the active constituents responsible for the diuretic activity, this study provides a basis for further exploration of its therapeutic potential.
CONCLUSION
The results of this study demonstrate the diuretic potential of the ethanolic extract of B. tomentosa roots. The observed increase in urine volume and electrolyte excretion supports its traditional use as a diuretic agent. The dose-dependent effect and comparable activity to furosemide indicate the potential utility of B. tomentosa roots in managing conditions associated with fluid overloads, such as edema, renal dysfunction, and hypertension. Further research is warranted to identify the active constituents and elucidate the mechanisms of action underlying the diuretic effect of B. tomentosa roots.
Acknowledgments: I would like to thank the staff of the Department of Pharmacology, Srinivas College of Pharmacy for their support.
Conflict of interest: None
Financial support: None
Ethics statement: The experimental protocol and the number of animals used in this research study were approved by the Institutional Animals Ethics Committee (IAEC) in its meeting held on 9th July 2022.