



|
Correlation between Cell Channels α-Helices Displacement and Frequency of Applied Electromagnetic Field Emanuele Calabrò 1,2*, Salvatore Magazù 1-5 |
|
1 Department of Mathematical and Informatics Sciences, Physical Sciences and Earth Sciences of Messina University, Messina, Italy. 2 CISFA - Interuniversity Consortium of Applied Physical Sciences, 98123 Messina, Italy. 3 Le Studium, Institute for Advanced Studies, Orléans, France. 4 Centre de Biophysique Moleculaire (CBM), rue Charles Sadron, Orleans, France; Laboratoire Interfaces, Confinement, Matériaux et Nanostructures (ICMN) - Université d’Orléans, 1b rue de la Férollerie, Orléans, France. 5 Istituto Nazionale di Alta Matematica "F. Severi" – INDAM – Rome, Italy. |
Abstract
In this study, the correlation between the displacement of the α-helices in cellular membrane channels and the frequency of the applied electromagnetic field was evaluated comparing the results after exposure to static, 50 Hz and 900 MHz electromagnetic fields of neuronal-like cells at the same intensity of 10 μT, using FTIR spectroscopy. The main result of this study was represented by the significant increase of the Amide I band, which was strictly related to the frequency of the field. Indeed, the integrated area of the Amide I band increased with increasing frequency, showing that the alignment of α-helices along the direction of the electromagnetic field is proportional to its frequency. This result can be applied in medicine, for instance, searching resonant natural frequencies of membrane channels in tumor cells and irradiating them by electromagnetic fields at those frequencies in order to damage their cellular functions. Also, it can be applied in modern technology, in order to plan technical wireless networks at frequencies far from resonant natural frequencies of biological systems.
|
Key Words: cellular membrane channels; alpha-helix; electromagnetic field; resonance; FTIR spectroscopy. |
INTRODUCTION
Cellular membrane is a selective flexible bilayer composed of phospholipids and proteins that separates the inner content (cytoplasm) of a cell from the external substance (plasma membrane).
Cellular channel proteins possess several characteristics of typical proteins [1-3]. They are embedded within the cell membrane and their primary function is to modulate the ions flux (Na+, K+, Ca2+, Cl−) across the cellular membrane, establishing a resting membrane potential and a concentration gradient.
Ions flux allows the maintenance of equilibrium between the membrane layers by opening and closing of these channels in response to external stresses such as temperature, pH changes, or mechanical forces [4-6] or electromagnetic fields [7-9]. Hence, ions flux is of fundamental significance in the cell functions.
Ion channels may be considered passive conductors so that the inside and outside of the cell should be out of equilibrium in order that ions flow across the channel. The force that drives ions flux is represented by the sum of an electrical transmembrane potential and ions’ concentration gradient across the membrane gives rise to an electrochemical gradient, whose range is generally from −55 to −70 mV. It is created by differences between the concentrations of sodium and potassium ions in the cytoplasm and in the plasma membrane, produced by opening and closing of channels, which induces a positive and negative charge influx into the cell until the balance is reached [10].
The opening and closing activities of channels are induced by physical or chemical signals. Given that the ions that move across cell channels are charged molecules, their flux density depends on the voltage difference between the inner and outer layers of the cellular membrane.
The cell channels in the cellular membranes are constituted by proteins, i.e. α-helices [1-3, 11] and amino acids whose interaction with an electromagnetic field is not negligible [12, 13]. In particular, α-helix proteins have a large dipole moment so that voltage-gated cation channels and their opening occur due to change in cellular membrane potential, which allows ions flux across them [14].
Interestingly, previous studies have shown that the diameter of cell channels can be changed by external stresses, allowing a change of ions flux in cell channels [15-17]. In particular, it was demonstrated that the exposure of proteins in bidistilled H2O solution to an electromagnetic field (EMF) induces the displacement of α-helices towards the applied EMF due to the large dipole moment of α-helix [18-20].
A direct consequence of such alignment could be represented by an enlargement of the diameter of cell membrane channels, which should cause the increase of ions flux through the cellular membrane following the second Ohm’s law [7-9]. This change of ions flux should alter the delicate equilibrium of cellular functions, inducing cell damage. Otherwise, change of ions flux in cellular membrane channels induced by exposure to EMFs was already largely observed in previous studies [21-26].
In this study, a correlation was observed between the rate of the displacement of α-helices in cell channels and the frequency of an applied EMF. This can be considered a relevant result, given that it can be applied in medicine to alter the cellular functions of tumor cells at their resonant frequencies and in modern technology, hypothesizing to plan wireless networks at frequencies far from resonant natural frequencies of biological systems.
MATERIALS AND METHODS
Materials
Samples of differentiated human SH-SY5Y cell line grown in 25-cm2 culture flasks were prepared following the protocol accurately described by Calabrò et al. 2013a, 2013b.
Experimental Design
In the first section of the experiment, the exposure system consisted of two Helmholtz coils that were used to generate a uniform magnetic field in the region around the center of the distance between the coils, at frequencies of 0 and 50 Hz. A Model 75 arbitrary function generator (Wavetek, Plainview, NY) and a 7570 AE power amplifier (Techron, Elkhart, IN) were used to drive the Helmholtz coils in order to generate a direct current (DC) and a static magnetic field (SMF) in the region between the coils in which neuronal-like cells were located. Also, a time-varying electromagnetic field was generated at the frequency of 50 Hz using an alternate current (AC) voltage linked to the Helmholtz coils, following the protocol described in [27, 28]. In the second part of the experiment, microwaves (MWs) at 900 MHz emitted by a mobile phone Samsung model GT-E1270 were used to irradiate neuronal-like cells, following the protocol in [29, 30]. The average specific absorption rate (SAR) that was computed during exposure was 0.2 W/kg, which was less than the limits of 1.6 and 2 W/kg recommended in the USA and Europe, respectively. In addition, this value is well within the limits specified by DIN VDE0848 standards and ANSI C95.1 [31] so that the exposure condition could be safely assumed to be non-thermal.
SMF and 50 Hz EMF were monitored by a magnetic field probe GM07 gaussmeter and MWs average intensity was monitored by SRM-3000 instrument of Narda Safety Test Solutions.
In order to compare the results of exposure as a function of frequency, the average intensity of the magnetic field component of EMF was 10 μT in all three exposures, in order to make it possible to compare the exposures.
The exposed samples were placed between the Helmholtz coils in an incubator (series 5400-115V models, Thermo Electron, Winchester, VA) with 5% CO2/95% humidity and at 37.0 + 0.1 °C, whilst unexposed cell samples were located into another incubator at the same physical conditions. However, the temperature was also monitored around the culture medium with an accurate Pt100 probe, using a handheld CTH 6200 thermometer (WIKA Wiegand, Klingenberg, Germany), which was already used in previous studies by researchers.
Infrared Spectroscopy
Exposed and unexposed samples of neuronal-like cells were treated with trypsin (0.05%) for 1 min, fixed with 70% ethanol, and cells adhered to glass coverslips following the protocol of [32] in order to be subjected to infrared (IR) spectroscopic techniques by FTIR spectrometer Vertex 80v of Bruker Optics. Spectra were acquired by collecting 128 interferograms using a spectral resolution of 4 cm-1. Reduction of residual water vapor, smoothing correction for atmospheric water background, and baseline correction were performed, following the protocol in [9].
Statistical analysis
The student’s T-test was applied to 15 neuronal-like cell samples and the experiment was repeated three times. The results below reported were considered significant with p < 0.05 for SMF, 50 Hz EMF, and 900 MHz MWs exposures with respect to control samples.
RESULTS AND DISCUSSION
FTIR spectroscopy was carried out using neuronal-like cell samples after 3 hours of separated exposures to SMF, 50 Hz, and 900 MHz EMFs at the intensity of 10 μT. Representative spectra of the neuronal-like cells after exposure to SMF, 50 Hz, and 900 MHz are reported in Figure 1 and are represented in orange, purple, and red colors, respectively, whilst the spectrum of representative unexposed samples is represented in black color.
The most relevant band that appeared in the spectra was the Amide I vibration band, centered around 1650 cm-1. It was due to C=O stretching and N―H bending vibrations and corresponds to α-helix proteins that are the most important feature of the secondary structure of proteins.
A relevant increase in the intensity of the Amide I vibration occurred after exposures to 10 mT electromagnetic fields at 0 and 50 Hz, 900 MHz, with p < 0.05 significance, which increased with increasing frequency, despite the intensity of the used EMF was the same for the three exposures. The increasing intensity of the Amide I band was already found in typical proteins in bidistilled water solution exposed to high frequency (HF) EMF. The alignment of α-helix proteins towards the applied EMF was used to explain this result, due to the torque effect caused by EMF [8, 18, 20]. It can be noted that in this study, the integrated area of the Amide I band increased significantly with the increasing frequency of applied EMF, showing a correlation between α-helix proteins displacement and the frequency of applied EMF.
This result could appear strange because we know that the acceleration of an electric charge subjected to EMF should be proportional to the strength of the applied field, according to the second law of dynamics. However, in order to schematize the response of cells’ α-helix to an applied EMF, our model can be represented by a macromolecule having a large dipole moment due to the circumstance that all the hydrogen bonds are directed towards the same direction because the peptide units of α-helix are oriented along its axis, giving a negative and positive charge at the carboxyl end and the amino end of the α-helix, respectively [33]. In addition, α-helices in the cellular membrane channels can be considered embedded in a viscous medium. The EMF to which samples were subjected can be represented by a complex function E= Eoejωt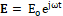 , where Eo and ω are the amplitude and the angular frequency of the EMF, respectively.
, where Eo and ω are the amplitude and the angular frequency of the EMF, respectively.
Hence, in order to explain the experimental result of this study, the equation of the motion of α-helical cell channels in a viscoelastic system under the application of a sinusoidal EMF should be considered.
The first term to be taken into account is the force induced by the EMF on a charge q, which represents a negative or positive charge depending on whether the carboxyl end or the amino end of the α-helix is considered, that is F = qE. In order that the displacement of α-helical cell channels occurs, this force should overcome the sum of the following forces.
First, the molecular bonding, which binds α-helix to the walls of cell channels. Indeed, the geometry of transmembrane α-helices surrounding the central pore of cell channels is influenced by H-bonding residues electrostatic and Van der Waals forces [34, 35]. Also in the mitochondrial membrane, the α-helix in voltage-dependent anion channel (VDAC) is linked to the inner wall of the channel by disulfide bonds [36, 37]. However, molecular bonding acting on the transmembrane α-helices can be represented by a spring element whose elastic force is given by the expression Fe = -mωs2x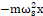 , where ωs is a resonant natural frequency of the α-helix, m and x are its mass and displacement, respectively, and k is the spring element of the system, which represents the binding type.
, where ωs is a resonant natural frequency of the α-helix, m and x are its mass and displacement, respectively, and k is the spring element of the system, which represents the binding type.
Second, the viscous force induced by the medium in the cell channels in which helices are embedded that can be written Fv=-mνdxdt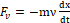 , where ν is the damper coefficient of the medium.
, where ν is the damper coefficient of the medium.
Finally, applying the material point approximation to a transmembrane α-helix, the generic equation of motion is given by [38]
md2xdt2=qE-mνdxdt-mωs2x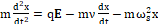 (1),
(1),
whose generic solution is x=x' ej(wt-φ) 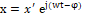 , in which x'
, in which x'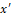 and φ are the amplitude of displacement and the phase angle, respectively.
and φ are the amplitude of displacement and the phase angle, respectively.
The amplitude of α-helix displacement can be found as follows:
x'= qmEωs2-ω2+jων 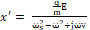 (2)
(2)
Looking at Eq. (2) we can see that the amplitude x'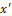 of the displacement of α-helix increases largely if the frequency of the field (ω) is close to a natural frequency of the system (ωs), producing a resonance phenomenon.
of the displacement of α-helix increases largely if the frequency of the field (ω) is close to a natural frequency of the system (ωs), producing a resonance phenomenon.
In this scenario, the assumption that the amount of dipole moment of α-helices in the cell channels increased due to the alignment of cells’ α-helices towards the applied field, can easily explain the observed result that the Amide I mode increased in intensity with the increasing frequency of EMF, such as it was already shown after exposure to EMF of proteins in bidistilled H2O solution [8, 9, 18, 20]. Hence, the scheme of the behavior mechanism of the cell channels under exposure to HF-EMF, which was proposed by the authors in their study where typical proteins were exposed to HF-EMFs (see Fig. 6-A and 6-B of [8]) was confirmed in this study by exposure of neuronal-like cells to EMFs at different frequencies. This finding is in agreement with previous results concerning the molecular mechanism of opening K+ channel that demonstrated that the inner α-helix should rotate by an angle of about 5-10 degrees during this process [35, 39, 40], providing a proof that the α-helical cell channels move within the channel during the gating process. Given these results, it is plausible to assume that the application of EMF to the cell membrane can alter the displacement of α-helices in the cell channels due to the large α-helix dipole moment [33, 41, 42] explaining the experimental findings of this study.
The competition between the impulse of applied EMF and the forces due to molecular bondings and cellular viscous medium should alter the delicate equilibrium in the cell membrane channel, inducing the enlargement of cellular channels such as already proposed in [8, 18]. The conditions in which the α-helical cell channels have a large dipole moment implies that the impulse of an applied EMF generates a torque on the α-helices that has to be compared with the average angular momentum from thermal agitation 2IαkT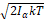 , where Iα is the moment of inertia of α-helix. Given that the moment of inertia of a generic α-helix should be less than the moment of inertia of a protein in which some α-helices are present, the result of calculation reported in [20] can be transferred to this study, confirming that the impulse of an applied EMF can overcome the average angular momentum from thermal agitation in cellular membrane, provided that an exposure time of some hours to the EMF is used, such as accurately explained in [8].
, where Iα is the moment of inertia of α-helix. Given that the moment of inertia of a generic α-helix should be less than the moment of inertia of a protein in which some α-helices are present, the result of calculation reported in [20] can be transferred to this study, confirming that the impulse of an applied EMF can overcome the average angular momentum from thermal agitation in cellular membrane, provided that an exposure time of some hours to the EMF is used, such as accurately explained in [8].
Interestingly, the correlation between the α-helix displacement and the frequency of EMF that can be explained mathematically by the decreasing of the term |ωs2-ω2|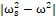 in Eq. (2), leads us to hypothesize that natural frequencies of the cell channels α-helices would be in the range of (or upper than) 900 MHz. Also, this finding is in agreement with previous results in which it was shown that natural resonant frequencies of biological systems can appear in the MWs range [43-46].
in Eq. (2), leads us to hypothesize that natural frequencies of the cell channels α-helices would be in the range of (or upper than) 900 MHz. Also, this finding is in agreement with previous results in which it was shown that natural resonant frequencies of biological systems can appear in the MWs range [43-46].
In this regard, the maximum displacement of the α-helical membrane channels exposed to EMF should occur at natural resonant frequencies. As a result, an important future application can be the treatment of tumor cells by using EMFs at resonant natural frequencies of their membrane channels, inducing cancerous cells death because of the change of ions flux through cellular channels caused by α-helices displacement. Indeed, using the continuum electrodiffusion modeling of Nernst–Planck equation, ions flux across cells membrane channels can be quantify combining Ohm’s law with Fick’s law of diffusion [10, 14]
I=D∇cL+gΔVLπ ro2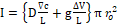 (3),
(3),
where I is the electric current represented by the ions flux, D the diffusion of the ions coefficient, g the channel conductivity, ro the average radius of the channel at rest (without the application of any force), and L is its length.
Hence, the radius of the channel can be calculated by adding the radius at rest ro and the displacement of the radius r'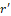 induced by exposure to EMF, which can be calculated by Eq. 2:
induced by exposure to EMF, which can be calculated by Eq. 2:
r= ro+ r'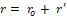
r'= qmEωs2-ω2+jων 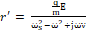 (4).
(4).
From the Eq. 3 and Eq. 4, we can conclude that the radius of cell channel r should increase with decreasing the term |ωs2-ω2|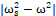 at resonance, inducing the increasing ions flux I across the cell membrane.
at resonance, inducing the increasing ions flux I across the cell membrane.
In this scenario, interesting applications in medical topics could be obtained, given that previous studies showed that change of ions’ flux through the cellular membrane channels of tumor cells regulates the initiation and progression of the disease [47-50].
CONCLUSIONS
The result of the exposure of neuronal-like cells to static, 50 Hz and 900 MHz EMFs at the same intensity of 10 mT was analyzed by means of FTIR spectroscopy. The main finding of this study was represented by the increasing Amide I vibration band, which increased with increasing EMF frequency. This result showed that the alignment of the α-helical cell channels towards the EMF occurred after exposure as a function of EMF frequency.
This result can be applied in medicine, for instance, searching resonant natural frequencies of α-helical cells membrane channels of tumor cells in order to irradiate them by EMFs at those frequencies to damage their cellular functions. Also, this result can be applied in modern technology, in order to plan technical wireless networks at frequencies far from resonant natural frequencies of biological systems.
Disclaimers
No financial support was received to carry out this study.
Compliance with Ethical Standards
Conflict of interest statement. The authors declare that they have no conflict of interest.
Research involving human participants and/or animals. The article does not contain any studies with human or animal participants. Hence, ethical approval is not applicable.
Informed consent. No study with humans or animals was carried out. Informed consent is not applicable.
ACKNOWLEDGMENTS
Many thanks to Prof. Riccardo Ientile (University of Messina, Italy) for his precious support in the preparation of SH-SY5Y cell samples.
REFERENCES