



|
Characterization and Antimicrobial Efficacy of Bovine Dermcidin-Like Antimicrobial Peptide Gene Nevien M. Sabry 1, Tarek A. A. Moussa 2* |
|
1 Cell Biology Department, Genetic Engineering and Biotechnology Division, National Research Centre, Giza 12622, Egypt. 2 Botany and Microbiology Department, Faculty of Science, Cairo University, Giza 12613, Egypt. |
ABSTRACT
Due to the significance of the antimicrobial peptides as innate immune effectors, in this study, a novel bovine antimicrobial peptide and its antimicrobial spectrum were described. RNA isolation from various tissues and RT-PCR were conducted. The DCD-like peptide was synthesized, and its antimicrobial effect was analyzed. The bovine dermcidin-like gene contains 5 exons intermittent by 4 introns. Bovine DCD-like mRNA was 398 bp with ORF size of 336 bp. Bovine DCD-like was expressed in skin and blood. Analysis of amino acid compositions showed that cysteine was repeated six times, which indicates the presence of 3 disulfide bonds that play a role in the peptide stability. Bovine DCD-like had an antimicrobial effect on Enterococcus faecalis, Streptococcus bovis, and Staphylococcus epidermidis, which was highest at 50 and 100 µg/ml. The effect on Candida albicans and Escherichia coli was slightly low. In Staphylococcus aureus, the activity of bovine DCD-like was affected greatly at pH 4.5 and 5.5. The optimum salt concentrations were 50 and 100 mM with E. coli and all other bacterial strains, respectively. In C. albicans, the activity of bovine DCD-like decreased with increasing pH regardless of the concentration of NaCl. The pH 6.5 of the sweat buffer was optimum for the activity of bovine DCD-like. Finally, it was concluded that the bovine DCD-like gene expressed in skin cells and the DCD-like peptide secreted into the sweat, had high antimicrobial activities against many Gram-positive and -negative strains, as well as yeast-like fungus.
Key Words: antimicrobial activity, RT-PCR, bovine dermcidin, skin, Bos Taurus.
INTRODUCTION
Antimicrobial peptides (AMPs) are considered as important innate immune defense molecules with diverse species that protect epithelial barriers. Many antimicrobial peptides displayed a broad-spectrum antimicrobial activity against various pathogens including fungi, bacteria, and viruses [1-3]; they also aid in wound healing and act as anticancer. [4, 5] The antimicrobial peptides exist in all organisms from humans to bacteria. They have been conserved through evolution across different species with many characteristics of separate AMPs classes. Due to their high potential and small size, they have been favored through evolution. [6, 7] The induction of peptides pathways in the insects, plants, and vertebrates are highly conserved. [8, 9] Generally, AMPs are secreted in animals at sites in contact with microbes, like mucosal epithelial cells or skin cells (genitourinary, gastrointestinal, respiratory, oral, etc.). AMPs in the animal can be secreted constitutively or as an infection response. [10]
Ruminant animals (e.g. goats, sheep, cattle, etc.) have a huge number of AMPs functioned as natural innate barriers limiting microbial infectious diseases in severity and proceed as a vital component in reaction to microbial infectious diseases. [11, 12] These peptides vary in size and mechanisms of action. AMPs have 2 groups: 1) cationic AMPs originate in all domestic animals, and 2) anionic AMPs that are a small group in ruminants, rich in glutamic and aspartic acids, and their antimicrobial activity are against Gram-positive and -negative bacteria [13]
Over 1500 antimicrobial peptides have been described, [14-17] and are assessed as possible alternatives to conventional antibiotics. Moreover, the amphoteric characteristics of many AMPs increase their permeation into the cell membrane lipids leading to their destruction and cell death. [18, 19]
Anomalous and notable for AMPs, the DCD-1L antimicrobial spectrum is preserved in a wide pH range and even at high salt concentrations, like in the human sweat conditions. [20] This significant activity suggests that the DCD-1L mechanism may be functionally different from most other AMPs. Some studies showed the DCD-1L binds to the bacterial surface and interacts with phospholipids of the bacterial membrane. [21, 22]
In this paper, we will describe a bovine dermcidin-like antimicrobial peptide secreted by skin cells and the spectrum of antimicrobial activities against fungi and Gram-positive and -negative bacteria.
MATERIALS AND METHODS
Samples collection
Different tissues including blood, kidney, intestine, muscle, liver, spleen, and skin were collected from Bos taurus. All tissue samples were collected using Allflex Tissue Sampling Units (TSU). All samples were frozen immediately in liquid nitrogen and transferred to -80°C once back to the lab till used.
RNA expression analysis of dermcidin
RNA was isolated from different tissues using TriFastTM (Peqlab, Erlangen, Germany). Then, PCR was performed using cDNA from different bovine tissues. The reaction mixture of PCR contained 1X Taq buffer, 0.4 mM dNTPs, 0.4 µM of each primer, 5 µl of the template, and 0.5 µl of Taq-polymerase (Fermentas, Germany). The used primers were 5ʹ-GACACACTAGAGACCAGAATCTCC-3ʹ and 5ʹ-TCAAAACATCTGTCCTCCCAC-3ʹ, producing a product with a size of ~400 bp. The PCR conditions were denaturation at 95°C for 4 min, then 35 cycles at 95ºC for 1 min, 58ºC for 1 min, and 72ºC for 80s, and the final extension at 72°C for 10 min. The PCR reaction was repeated triple with two negative and one positive control. A ladder of 100-bp (Fermentas, Germany) was used in agarose gel electrophoresis.
Antimicrobial assay
Bovine DCD-like peptide was synthesized by FlexPeptide™ Technology (GenScript, USA). The antimicrobial activity of Bovine DCD-like was analyzed as described by Valore et al. [23] using Gram-positive bacteria included Enterococcus faecalis (ATCC29212), Staphylococcus epidermidis (ATCC1228), methicillin-resistant Staphylococcus aureus (MRSA) (ATCC43330), Streptococcus bovis (ATCC49147); while G-ve bacteria included Escherichia coli (ATCC25922), and the fungus Candida albicans (ATCC1021). E. coli was grown in LB medium, S. epidermidis in Nutrient medium, S. bovis in Trypticase soy medium with defibrinated sheep blood, S. aureus and E. faecalis in Columbia medium, and C. albicans in Casein hydrolysate medium. The concentrations of bacterial and yeast strains were determined spectrophotometrically. Bacteria and yeast strains were diluted to 2X106 CFU/ml in phosphate buffer. The cells were incubated in 200 µl of phosphate buffer at 37ºC for 4 h with different concentrations of synthesized peptide or sweat fractions. The microbial cultures were diluted 1:50-500, and 50 µl and 100 µl of each of the solutions were plated in duplicate on agar plates. At least five plates were evaluated from each experiment, and then the mean number of colonies was calculated. The bactericidal activity of the reagents tested was represented by the percentage of killed cells and was expressed as:
Bactericidal activity=1-number of cells survival after peptide incubationnumber of cell survival after control peptide incubation× 100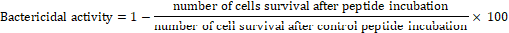
The activity profile was performed by incubating the test organisms with 10 µg/ml of the synthesized bovine DCD-like or sweat fraction DCD-like for 4 h in 10 mM phosphate buffer at different pH values (4.5, 5.5, 6.5, or 7.5), sodium chloride (25, 100, or 150 mM), and sweet buffer (10 mM KCl, 40 mM NaCl, 1.0 mM CaCl2, 1.0 mM MgCl2, and 1.0 mM NaH2PO4) at pH 5.5 or 6.5.
RESULTS AND DISCUSSION
The discovery of antimicrobial peptides in animals is expanding and advances in this field are expected. It will be likely to find new genes that encode antimicrobial peptides in sequenced animal genomes. [24]
The discovered bovine dermcidin gene has not been published previously, we detected the sequence and locus of this gene using in silico determination based on conserved synteny between bovine chromosome 5, human chromosome 12, monkey chromosome 11, and chimpanzee chromosome 12. [25-27] We detected the sequence in the cattle genome by the alignment of the human dermcidin gene with the cattle genome, and found the bovine DCD-like gene sequence by analyzing the segment (Figure 1 and Table 1).
Table 1: Comparative genome map of Chromosome 12 (Homo sapiens), Chromosome 12 (Pan troglodytes), Chromosome 11 (Macaca mulatta), and Chromosome 5 (Bos taurus), the compared segments has been mentioned between brackets.
|
Organism |
Gene |
Locus |
|
|
Name |
Abbreviation |
|
|
|
Homo sapiens (Human) |
Neuronal differentiation 4 |
NEUROD4 |
Chromosome 12, NC_000012.12 (55019945..55030017) |
|
Pan troglodytes (Chimpanzee) |
Neuronal differentiation 4 |
NEUROD4 |
Chromosome 12, NC_036891.1 (34055442..34065514, complement) |
|
Macaca mulatta (Monkey) |
Neuronal differentiation 4 |
NEUROD4 |
Chromosome 11, NC_027903.1 (53721646..53731727) |
|
Bos taurus (Cattle) |
Neuronal differentiation 4 |
NEUROD4 |
Chromosome 5, NC_037332.1 (59882602..59920743, complement) |
|
Homo sapiens (Human) |
Thymocyte expressed, positive selection associated 1 |
TESPA1 |
Chromosome 12, NC_000012.12 (54948019..54984746, complement) |
|
Pan troglodytes (Chimpanzee) |
Thymocyte expressed, positive selection associated 1 |
TESPA1 |
Chromosome 12, NC_036891.1 (34100708..34136106) |
|
Macaca mulatta (Monkey) |
Thymocyte expressed, positive selection associated 1 |
TESPA1 |
Chromosome 11, NC_027903.1 (53651281..53686091, complement) |
|
Bos taurus (Cattle) |
Thymocyte expressed, positive selection associated 1 |
TESPA1 |
Chromosome 5, NC_037332.1 (59925012..59960265) |
|
Homo sapiens (Human) |
Mucin like 1 |
MUCL1 |
Chromosome 12, NC_000012.12 (54854515..54858393) |
|
Pan troglodytes (Chimpanzee) |
Mucin like 1 |
MUCL1 |
Chromosome 12, NC_036891.1 (34226741..34230956, complement) |
|
Macaca mulatta (Monkey) |
Mucin like 1 |
MUCL1 |
Chromosome 11, NC_027903.1 (53526781..53530653) |
|
Bos taurus (Cattle) |
Mucin-like 1 |
MUCL1 |
Chromosome 5, NC_037332.1 (25215043..25220249, complement) |
|
Homo sapiens (Human) |
Dermcidin |
DCD |
Chromosome 12, NC_000012.12 (54644591..54648493, complement) |
|
Pan troglodytes (Chimpanzee) |
Dermcidin |
DCD |
Chromosome 12, NC_036891.1 (34435763..34439668) |
|
Macaca mulatta (Monkey) |
Dermcidin |
DCD |
Chromosome 11, NC_027903.1 (53338985..53343039, complement) |
|
Bos taurus (Cattle) |
Dermcidin |
DCD |
chromosome 5, NC_037332.1 (25435277.. 25437603) |
|
Homo sapiens (Human) |
Lacritin |
LACRT |
Chromosome 12, NC_000012.12 (54630839..54634879, complement) |
|
Pan troglodytes (Chimpanzee) |
Lacritin |
LACRT |
Chromosome 12, NC_036891.1 (34449360..34453441) |
|
Macaca mulatta (Monkey) |
Lacritin |
LACRT |
Chromosome 11, NC_027903.1 (53324690..53328815, complement) |
|
Bos taurus (Cattle) |
Lacritin |
LACRT |
chromosome 5, NC_037332.1 (25446731.. |
|
Homo sapiens (Human) |
glycosylation dependent cell adhesion molecule 1 |
GLYCAM1 |
Chromosome 12, NC_000012.12 (54608187..54610462, complement) |
|
Pan troglodytes (Chimpanzee) |
- |
- |
- |
|
Macaca mulatta (Monkey) |
- |
- |
- |
|
Bos taurus (Cattle) |
glycosylation-dependent cell adhesion molecule 1 |
GLYCAM1 |
Chromosome 5, NC_037332.1 (25478966..25481870) |
|
Homo sapiens (Human) |
protein phosphatase 1 regulatory inhibitor subunit 1A |
PPP1R1A |
Chromosome 12, NC_000012.12 (54579240..54588659, complement) |
|
Pan troglodytes (Chimpanzee) |
protein phosphatase 1 regulatory inhibitor subunit 1A |
PPP1R1A |
Chromosome 12, NC_036891.1 (34495553..34503977) |
|
Macaca mulatta (Monkey) |
protein phosphatase 1 regulatory inhibitor subunit 1A |
PPP1R1A |
Chromosome 11, NC_027903.1 (53271995..53281127, complement) |
|
Bos taurus (Cattle) |
protein phosphatase 1 regulatory inhibitor subunit 1A |
PPP1R1A |
Chromosome 5, NC_037332.1 (25506930..25514982) |
|
Homo sapiens (Human) |
phosphodiesterase 1B |
PDE1B |
Chromosome 12, NC_000012.12 (54549393..54579239) |
|
Pan troglodytes (Chimpanzee) |
phosphodiesterase 1B |
PDE1B
|
Chromosome 12, NC_036891.1 (34504986..34535264, complement) |
|
Macaca mulatta (Monkey) |
phosphodiesterase 1B |
PDE1B
|
Chromosome 11, NC_027903.1 (53241661..53271924) |
|
Bos taurus (Cattle) |
phosphodiesterase 1B |
PDE1B |
Chromosome 5, NC_037332.1 (25515721..25542387, complement) |
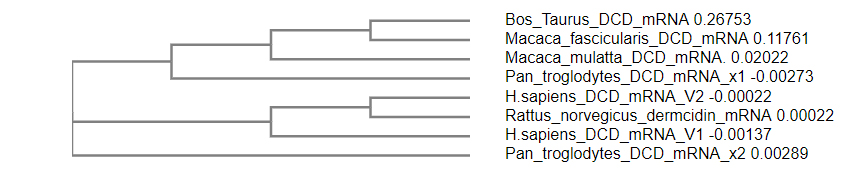
Figure 1: Phylogenetic tree showed the relation between the discovered bovine dermcidin-like mRNA (this study) and the other dermcidin genes in different organisms.
Analysis of bovine dermcidin-like nucleotides
The gene had no homology to any bovine gene sequence published, so it was designated as dermcidin-like (bovine DCD-like). The full length of the bovine DCD-like-cDNA product was sequenced and identified, with 398 bp that had ORF with a length of 336 bp (Figure 2). The full length of the dermcidin gene was 2205 bp, containing five exons intermittent by 4 introns varying in size, in which the exon 1 was 59 bp, exon 2 was 23 bp, exon 3 was 112 bp, exon 4 was 90 bp, exon 5 was 52 bp and finally 3 bp stop codon (Figure 2) when compared with the PCR fragment of Bovine DCD-like-cDNA sequence. All introns splice junctions corresponded to the GT–AG rule. The bovine DCD-like gene was deposited in Genebank with the accession number of AB932628.
The human DCD gene contained 5 exons and was expressed as a single transcript with a protein with 110 amino acids, with 19 amino acids as N-terminal signal peptide [20, 28] and was identified in humans for the first time as a gene expressed specifically in sweat glands. [20] The precursor protein with 110 amino acids is proteolytically processed in sweat, giving rise to many truncated DCD peptides vary in charge and length. [29-31]
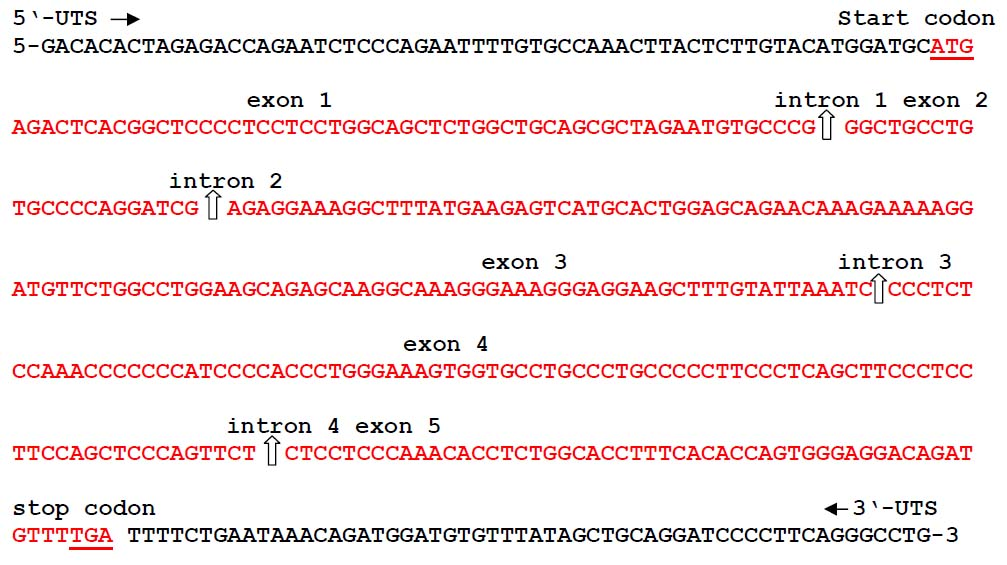
Figure 2: Structure of the bovine dermcidin-like gene (bovine DCD-like) secreted by the skin of Bos taurus and has the accession number of AB932628.
Analysis of bovine dermcidin-like peptide
The bovine DCD expression profile was determined by testing RNA from different tissues and it was found that bovine DCD-like was expressed in bovine skin tissue and blood only, while the expression was not detected in any other tissues (spleen, liver, muscle, intestine, and kidney) (Figure 3). This showed that the expression of bovine DCD-like was restricted to skin cells. The analysis of the amino acid sequence of Bovine DCD-like peptide indicated that it is composed of 18 different amino acids repeated to produce a peptide with 112 amino acids. The most abundant amino acids were proline and leucine (repeated by 15 times each) and the rarest amino acids were histidine, asparagine, and tryptophan (represented by one time each). Cysteine was repeated 6 times, which indicated that 3 disulfide bonds play a role in the stability of the peptide (Table 2). The peptide sequence was applied to the SignalP-4.1 online program (http://www.cbs.dtu.dk/services/SignalP/) to determine the signal peptide length that was the first 21 amino acids (Figure 4). Also, the predicted Bovine DCD-peptide sequence was applied to Compute pI/Mw (http://web.expasy.org/cgi-bin/compute_pi/pi_tool) to determine the theoretical isoelectric point and molecular weight of the peptide that was 9.24 for pI and 11.91 kDa for Mw.
Eccrine glands are the most developed and abundant glands in humans. They are distributed all over the body's surface. On the other hand, in cow, donkey, horse, camel, and canid species, apocrine glands are distributed all over the surface of the animal body and have evaporative cooling functions. Eccrine glands secret odorless sweat, mainly consisting of water, lactic acid, urea, ammonia, proteins, amino acids, and small traces of salts. Apocrine glands secrete sweat that consists of steroids, lipids, and proteins. [32] The expression of human dermcidin (DCD) is localized in skin cells, where is expressed in eccrine sweat glands constitutively, secreted into the sweat, and transported to the skin surface. [20]
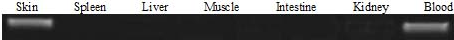
Figure 3: RT-PCR analysis of various tissues. As shown, bovine DCD-like expression was restricted to cells in the skin.
Table 2: Amino acids composition of the deduced Bos tautrus DCD-AMP
|
Amino acid |
Single letter |
Number |
Type of amino acid |
|
Methionine |
M |
3 |
Nonpolar (hydrophobic) |
|
Arginine |
R |
5 |
+ve |
|
Leucine |
L |
15 |
Nonpolar (hydrophobic) |
|
Threonine |
T |
4 |
Polar (hydrophilic) |
|
Alanine |
A |
12 |
Nonpolar (hydrophobic) |
|
Proline |
P |
15 |
Nonpolar (hydrophobic) |
|
Glutamic acid |
E |
7 |
-ve |
|
Cysteine |
C |
6 |
Polar (hydrophilic) |
|
Glycine |
G |
10 |
Nonpolar (hydrophobic) |
|
Isoleucine |
I |
2 |
Nonpolar (hydrophobic) |
|
Lysine |
K |
8 |
+ve |
|
Phenylalanine |
F |
5 |
Nonpolar (hydrophobic) |
|
Serine |
S |
8 |
Polar (hydrophilic) |
|
Histidine |
H |
1 |
+ve |
|
Glutamine |
Q |
7 |
Polar (hydrophilic) |
|
Asparagine |
N |
1 |
Polar (hydrophilic) |
|
Tryptophan |
W |
1 |
Nonpolar (hydrophobic) |
|
Valine |
V |
2 |
Nonpolar (hydrophobic) |
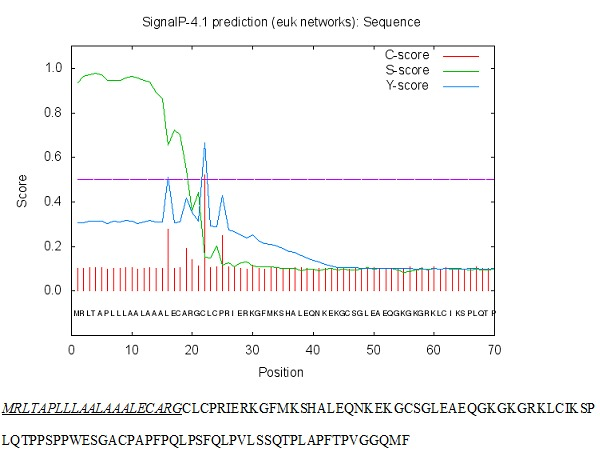
Figure 4: The signal peptide length was determined using online SignalP-4.1 euk predictions (http://www.cbs.dtu.dk/services/SignalP/).
Antimicrobial activity of the bovine dermcidin-like peptide
The synthesized Bovine DCD-like peptide had a broad antimicrobial activity against gram +ve, gram -ve bacteria, and yeast fungus. For G +ve bacteria, the most affected species were S. epidermidis, S. bovis, and E. faecalis, respectively, in which the antimicrobial effect increased with increasing peptide concentration. The highest antimicrobial effect was achieved at 50 and 100 µg/ml, but no significant difference was observed between the two concentrations in terms of effect. In this study, the methicillin-resistant S. aureus (MRSA) exhibited the same behavior but the maximum antimicrobial effect was achieved at 50 µg/ml, and then it was reduced at 100 µg/ml. For G –ve bacterium (E. coli) the antimicrobial effect was slightly less than G +ve bacteria. C. albicans affected the antimicrobial peptide, so that no effect was observed at 1 µg/ml and the inhibitory effects started at 5 µg/ml increase reaching the maximum at 100 µg/ml (Figure 5).
To study the antimicrobial efficacy of the Bovine DCD-like peptide, we evaluated the antimicrobial activity using different pH values with or without NaCl concentrations. In all bacterial strains, the varying pH values did not affect the activity of Bovine DCD-like peptide, except S. aureus, in which the antibacterial activity highly affected at pH 4.5 and 5.5, where the cell death percentage was less than 30% (Figure 6). In the presence of NaCl concentrations, the antimicrobial activity of Bovine DCD-like peptide did not change with salt concentrations, where 100 mM NaCl was the optimum concentration, which gave the highest antibacterial activity in all bacterial strains except E. coli in which the antibacterial activity was affected greatly after 50 mM NaCl concentration, and the optimum NaCl concentration was 50 mM NaCl. The behavior of C. albicans was contrary to the bacterial behavior, where the antimicrobial activity of Bovine DCD-like peptide decreased with increasing pH in the absence and presence of NaCl concentrations. In general, pH 6.5 of the sweat buffer was mostly suitable for increasing the activity of Bovine DCD-like peptide in all bacterial strains (Figure 6).
Anionic antimicrobial peptides are a small group present in ruminants, which are rich in glutamic and aspartic acids and have general antimicrobial activity against Gram-positive and -negative bacteria. [13, 33] The fraction of DCD-like peptide in sweat has an antimicrobial spectrum against various pathogenic microorganisms. Cattles have apocrine sweat glands, each hair fiber is associated with one gland. [34, 35]
The major dermcidin peptide fragment in sweats is DCD-1L with 48 mers and has a net charge of -2 that is anionic and capable of killing/inhibiting microbes like methicillin-resistant S. aureus, isoniazid- and rifampin-resistant Mycobacterium tuberculosis, Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Listeria monocytogenes, Salmonella thyphimurium, Pseudomonas putida, and Candida albicans. [20, 28, 36, 37]
Cattle sweat has high contents of inorganic salt and total protein nitrogen and relatively high urea content. [38] The human DCD-1L has a wide range of antimicrobial activities over a high salt concentration and a wide pH range [39], which resembles the human sweat conditions. [20]
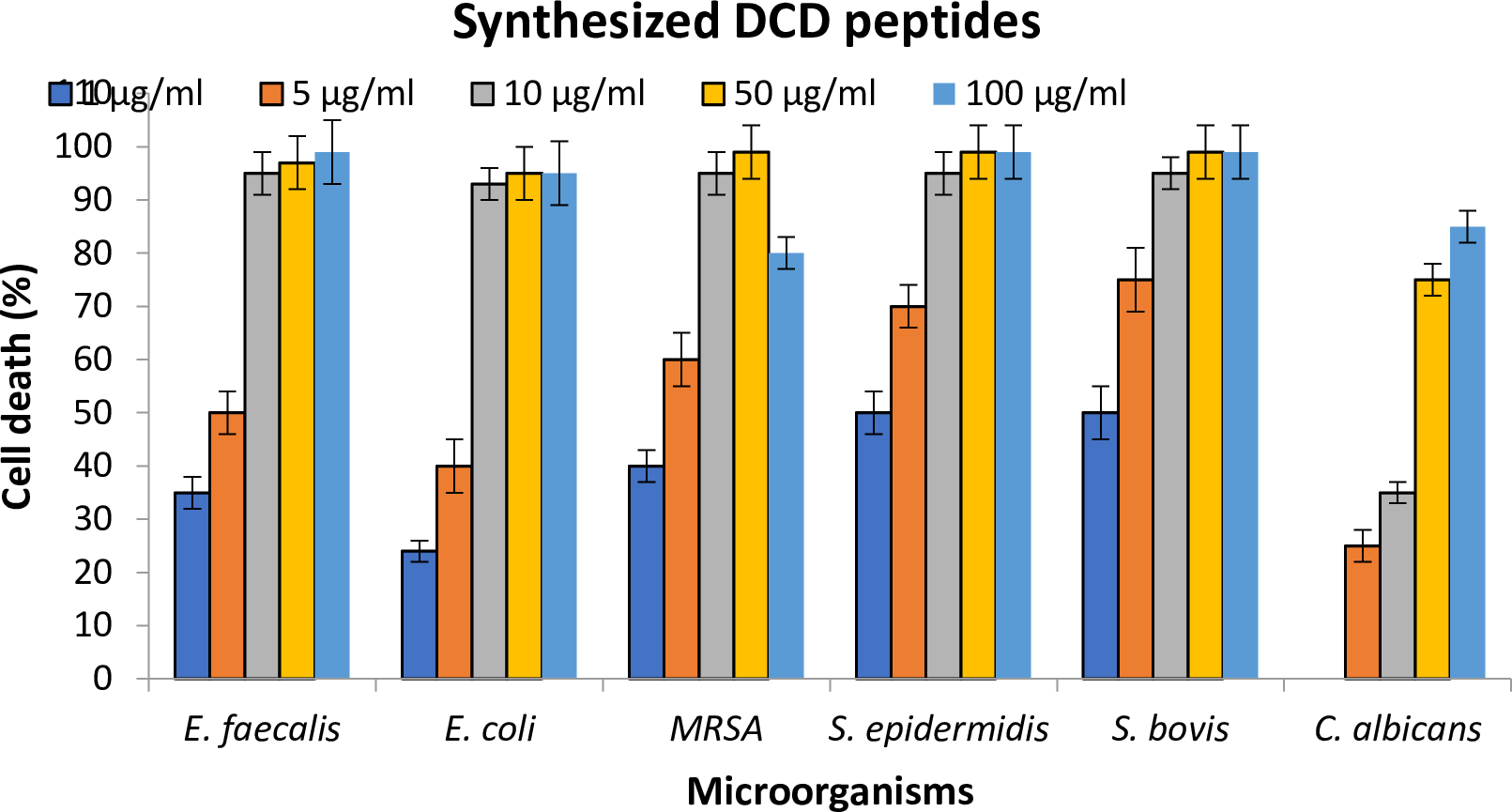
Figure 5. Antimicrobial efficacy (cell death) of various concentrations of synthesized Bovine DCD-like peptides against selected microorganisms.
|
|
|
Figure 6. Antimicrobial efficacy of the bovine DCD-like secreted from skin cells of Bos taurus on selected microorganisms, using different pH values of phosphate buffer, pH 7 of phosphate buffer amended with different concentrations of salt, and different pH values of sweat buffer.
CONCLUSION
The bovine DCD-like gene is expressed in skin cells and the bovine DCD-like peptide is secreted into the sweat and has high antimicrobial activities against Gram-positive and negative bacteria, as well as yeast-like fungus. The results revealed that the bovine DCD-like peptide activity was not affected by different salt concentrations; this means that the bovine DCD-like peptide has been adapted to work in all salt concentrations that are secreted in the sweat.
REFERENCES