



|
Characterization and Antibacterial Activity of Silver-Manganese Bimetallic Nanoparticles Biofabricated Using Arachis Pintoi Extract
Huynh Van Tien1, Nguyen Tri2,3*, Nguyen Phung Anh3, Dang Mai Nhi3, Le Thi Bich Hang2, Duong Nhat Linh2, Nguyen Van Minh2 |
|
1 Ho Chi Minh City University of Food Industry, Ho Chi Minh City, Vietnam 2 Ho Chi Minh City Open University, Ho Chi Minh City, Vietnam 3 Institute of Chemical Technology-VAST, Ho Chi Minh City, Vietnam. |
ABSTRACT
This is the first time the bimetallic Ag-Mn nanoparticles (Ag-Mn)NPs were synthesized using Arachis pintoia extract. The obtained samples were characterized and analyzed by various techniques, including X-Ray Diffraction, TEM, and FT-IR. XRD analysis confirmed the crystalline nature of nanoparticles. TEM images showed that the samples were nearly spherical in shape and uniform in size distribution with the average nanosizes of 3.3 nm for bimetallic (Ag-Mn)NPs, and 11.8 nm for monometallic AgNPs. FT-IR confirmed that flavonoids, phenolic acids, and alkaloid molecules can be bound to NPs acted both as the reducing and stabilizing agents. The results suggested that (Ag-Mn)NPs showed much greater potential against bacteria than that of AgNPs.
Key Words: Antibacterial, Ag-Mn, bimetallic, nanoparticles, Arachis pintoi.
INTRODUCTION
The research in nanotechnology pledges quantum leaps not only in materials manufacturing and electronics but also possesses a lot of applications in healthcare, medicine, energy, and biotechnology. The use of the plant, algae, fungi, and bacteria provides eco-friendly, safe, reliable, and economical routes to synthesize nanoparticles (NPs). However, plant-mediated synthesis of nanoparticles is more advantageous compared to the methods that use microorganisms because they can be easily improved, are less biohazardous and are not involved in the elaborate stage of growing cell cultures [1]. Plants’ parts like seeds [2], leaf [3], bark [4], stem [5] and fruit extracts [6] have been effectively used for synthesizing nanoparticles.
Metallic nanoparticles were classified as monometallic, bimetallic, and trimetallic nanopaticles. For preparation of bimetallic nanoparticles from precursor salts, two methods are adopted including co-reduction, and successive reduction [7]. Though a number of bimetallic nanoparticles have been synthesized through these processes, silver-manganese nanoparticles - (Ag-Mn)NPs are much less investigated although both are considered as antibacterial agents at the independent level. Manganese nanoparticles (MnNPs) have proven to be effective against bacteria. In the context of monometallic MnNPs, their easy fabrication, environment affable nature and the non-toxic means of synthesis make MnNPs one of the best alternatives for a number of biological uses. They possess antibacterial activity against broad spectrum microbes [8]. Green synthesis of MnNPs was reported in various publications. Employing plant extract as a reduction and stabilization agent of manganese metal into MnNPs is one of the simple, environment-friendly, and inexpensive approaches in green chemistry [8, 9]. Jayandran et al. [8] synthesized MnNPs by reducing manganese acetate using the lemon methanolic extract. The average crystallite size of nanoparticles was in the range of 50 nm. The antibacterial activities of MnNPs against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Staphylococcus bacillus were appraised. The results showed that the antibacterial activities were superior for all bacteria. On the other hand, the antifungal activities of Mn NPs were studied against four fungal strains including Trichophyton simii, Curvularia lunata, Aspergillus niger, and Candida albicans. The results showed high antifungal activity against all fungal strains. Meanwhile, silver nanoparticles (AgNPs) exhibit the most efficient antibacterial and antifungal properties [10-12]. Antibacterial activity along with the mechanism of action of silver nanoparticles on both gram negative and gram positive bacteria, including Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Streptococcus mutans, etc. has already been investigated [13]. The applications of both Mn and Ag nanoparticles validate the importance of both types of nanoparticles and require further exploration for the well-being of humans. However, no paper was published in the synthesis of (Ag-Mn)NPs through the green chemistry approach.
Arachis pintoi (A. pintoi) - a tropical legume has been originated from South America and has been widespread in the subtropics and wet tropics. It has been introduced to many areas including Argentina, Australia, Colombia and the USA, and countries in South-East Asia, Central America, and the Pacific. A. pintoi can be used as a ground cover as well as an ornament. A. pintoi is compatible with aggressive grasses such as Brachiaria and is tolerant of heavy grazing. Previous chemical analyses of the plant indicate the presence of phytosterols and flavonoids, phenolic acids, triterpenes, alkaloids, fatty acids, etc. [14]. These components can be used as a reducing agent in the preparation of nanoparticles. However, much less study of nanoparticle synthesis has been reported to date using A. pintoi extract as a combined reducing and stabilizing agent.
In this paper, Ag-Mn nanoparticles were synthesized by co-reduction of AgNO3 and KMnO4 solution using A.pintoi extract as a combined reducing and stabilizing agent. The physico-chemical properties of the obtained Ag-MnNPs were investigated. The antibacterial feature of silver nanoparticles was evaluated throughout against gam (-) bacteria including Escherichia coli (E. coli), Salmonella typhimurium (Salmonella), and Pseudomanas aeruginosa (P. aeruginosa); and gam (+) bacteria: Staphylococcus aureus (S. aureus) and Bacillus cereus (B. cereus).
MATERIAL AND METHODS
Preparation and Characterization of Sample
A. pintoi (both leaves and stems) were collected and washed several times with distilled water to eliminate the dust particles, and then ground to the fine powder in distilled water with the mass ratio of A. pintoi/water being 1/10. The mixture was boiled at 70 ºC for 2 hours to extract the reducing agents in the presence of leaves and stems of A. pintoi. The obtained extract was cooled to room temperature and then filtered with Whatman filter paper 0.22 mm before centrifuging at 5,000 rpm for 30 minutes to get rid of the heavy biomaterials. The extract was stored in the refrigerator at 4 ºC in order to be used for further experiments.
The AgNPs synthesis was performed by mixing silver nitrate (AgNO3, Merck, >99.8%) solution with A. pintoi extract under stirring at room temperature and illuminated by the sunlight. According to our unpublished work, the suitable conditions for the synthesis of AgNPs using A.pintoi extract as reducing and stabilizing agent were determined such as AgNO3 concentration of 1.75 mM, the volume ratio of AgNO3 solution/A. pintoi extract of 4.0/1.0, stirring rate of 300 rpm and the synthesis duration of 90 minutes. The synthesis of Ag-Mn nanoparticles was performed similarly to the above with the ratios of AgNO3 and KMnO4 solutions followed by 2:1 and 1:1. The obtained Ag-Mn nanoparticles were symbolized (1Ag-1Mn)NPs and (1Ag-2Mn)NPs, respectively.
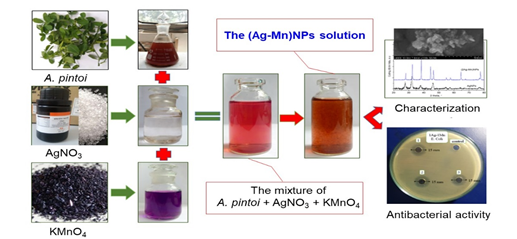
Figure 1. Illustration of the experimental process in biosynthesis of Ag-Mn bimetallic nanoparticles
The crystalline phases of the obtained AgNPs or (Ag-Mn)NPs powder which were washed and dried were determined by X–ray diffraction (XRD) using Bruker D2 Phaser powder diffractometer. The morphology of thesamples was characterized by transmission electron microscopy (TEM) using JEOL JEM 1400 instrument. A drop of the solution of samples was put on the carbon stub, dried and observed in TEM. The relation of nanoparticles with A. pintoi extract was investigated using Fourier transform infrared spectroscopy (FT-IR) carried out on a Tensor 27-Bruker spectrophotometer operating in the range of 400–4,000 cm-1 with resolution range of 2 cm-1.
Antibacterial Activity
The obtained samples were tested for antibacterial activity against E. coli, Salmonella, P. aeruginosa, S. aureus, and B. cereus by the zone of inhibition and minimum inhibitory concentration which awere also stated in our previous work [15]. The bacterial strains were cultured with the Mueller-Hinton Agar (MHA) method, and then the strains were stored in the agar rods at 4 oC. Three strains were added to the tube contained 5 mL pasteurized Trypticase Soy Broth (TSB) and statically cultured at 37 oC in 18 hours. The paper discs were wetted by the solutions and allowed to dry, these dried discs then were placed on the agar. All the samples were stored in the refrigerator for 8 hours and finally incubated at 37 oC for 18 hours. The antibacterial activity was recorded by measuring the diameter of the zone of inhibition around the agar.
RESULTS AND DISCUSSIONS
Characterization of the Synthesized Samples
The crystal structure of nanosamples was verified by XRD diffraction diagram (Fig.2). The results showed that the samples of AgNPs solution appeared at diffraction peaks at 38.1°; 46.1°, 64.1° and 76.9° corresponding to the plane (111), (200), (220) and (311) of the silver nanoparticles (JCPDS card No. 89-3722) [16]. In addition, a few intense and unassigned peaks at 2q = 28.1°, 32.4°, 55.6°, and 57.0° in the vicinage of silver peaks exhibited the presence of bio-organic phase crystals [17], which were present in A. pintoi extract and responsible for silver ions reduction and stabilization of nanoparticles. Besides the appearance of AgNPs peak, the XRD diffractions of Ag-Mn samples also appeared at diffraction peaks at 38.4°, 46.4°, 57.6°, 64.6° and 77.8° characterized by the planes (310), (330), (510), (611), (721) of MnO2 nanoparticles. On the other hand, the peaks at 2q = 44.5° and 55.0° were characterized by the manganese crystal. Obviously, the crystallinity of the Ag-Mn samples is much higher than the AgNP sample.
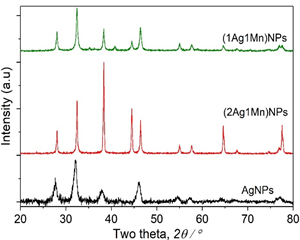
Figure 2. XRD patterns of the samples
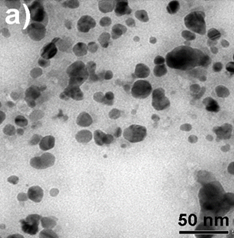
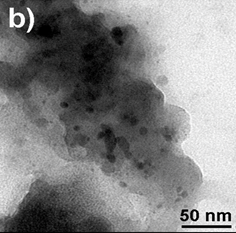
Figure 3. TEM images of AgNPs (a) and
(2Ag-1Mn)NPs (b) samples
TEM image provided further insight into the morphology and size details of the formed nanoparticles. The result of the TEM image of the AgNPs sample (Fig.3a) indicated that nanoparticles were highly dispersed with a spherical shape size ranging of 0-40 nm. TEM images of the (2Ag-1Mn)NPs sample (Fig.3b) also showed similar results. The images clearly showed a bio-organic layer coating around silver and manganese nanoparticles. This layer was due to surrounded phytochemicals in the extracts that served as a capping agent to prevent agglomeration. On the samples, silver and manganese nanoparticles mixed together had a relatively uniform size and were smaller than the pure AgNPs. Obviously, the sample (2Ag-1Mn)NPs has the smallest and most uniform particle size. From the TEM image, by using the ImageJ software, the size distribution of the samples was elucidated in Fig.4. The average sizes of AgNPs and (2Ag-1Mn)NPs were determined to be 11.8 and 3.3 nm, respectively.
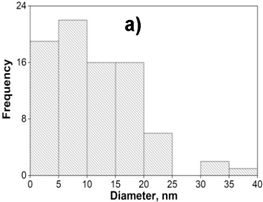
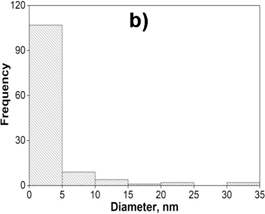
Figure 4. The size distribution histogram of AgNPs (a) and (2Ag-1Mn)NPs (b) samples
FT-IR spectra of A. pintoi extract, AgNPs solution, AgNPs powder, (1Ag-1Mn)NPs and (2Ag-1Mn)NPs powder were depicted in Fig.5. The spectrum for A.pintoi extract showed typical broad O-H stretching (3600-3300 cm-1) and C=O stretching (1730-1690 cm-1), suggesting the presence of flavonoids, phenolic acids, and alkaloids in extract that acted both as the reducing and stabilizing agents [18]. The difference was not much on the samples of pure A. pintoi extract and AgNPs solution. This could be because the compounds of A. pintoi extract covered the peaks of the silver nanoparticles by the functional groups contained in the extract, so the formation of the peaks of the silver nanoparticles was unclear. However, compared to pure A. pintoi extract, the FT-IR spectrum of AgNPs solution showed the presence of a weaker signals, proving the components of the extract reacted to form silver nanoparticles, so the concentration of the extract was significantly reduced. On the FT-IR spectrum of AgNPs and (2Ag-1Mn)NPs powder, a band centered at 2950 cm-1 was attributed to the axial stretching of C-H bonds, a peak at 1810 cm-1 was related to the axial stretching of C=O bonds of the acetamide groups, a peak at 1420 cm-1 corresponded to the symmetric angular deformation of CH3, the band range of 1340–1440 cm-1 was assigned to CH2 wagging and CH bending, the bands range of 1200–1300 cm−1 was associated with N–H bend amines and there was a broadband in the wavenumber range of 1153–897 cm-1 indicating the polysaccharide skeleton, including the vibrations of the glycoside bonds, C–O and C–O–C stretching. From the analysis of FT-IR studies, we confirmed that the flavonoids and alkaloids components together with phenolic acids in A. pintoi extract possibly performed dual functions for the formation and stabilization of AgNPs.
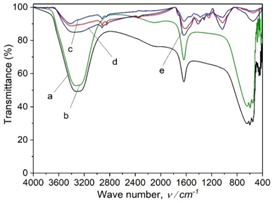
Figure 5. FT-IR spectra of A. pintoi extract (a), AgNPs solution (b), powder of AgNPs (c),
(1Ag-1Mn)NPs (d), and (2Ag-1Mn)NPs (e)
Antibacterial Activity
The antibacterial activity of the samples was determined against E. coli bacterial strain on an agar plate. According to our unpublished work, the A. pintoi extract did not show any antibacterial activity against all tested bacteria. Fig.6 showed the inhibition zone of the samples against the bacteri. The samples showed good antibacterial activity against E. coli. Specifically, the average diameters of antibacterial rings were 12.3, 16.0, and 15.0 mm, respectively for AgNPs, (2Ag-1Mn)NPs, and (1Ag-1Mn)NPs. The bimetallic nanoparticles showed better antibacterial activity than the pure AgNPs. This may be due to the nature of Ag which is highly dispersed in the MnO2 matrix, so that Ag can be more exposed to bacterial cells and shows potent antibacterial activity compared to bulk silver or AgNPs alone. Moreover, the advantage of incorporation of Ag into MnO2 lattice is the reduction of toxicity of free ions to human cells [19]. Obviously, the (2Ag-1Mn)NPs sample was the most outstanding. Appreciable antibacterial activity of (2Ag-1Mn)NPs is due to its nanosize and Mn4+; whereas, the slightly lower antibacterial activity of (1Ag-1Mn)NPs is accredited to its higher nanosize and lower Mn4+ oxidation state in the sample as revealed by TEM and XRD analysis. Therefore, (2Ag-1Mn)NPs sample was chosen to investigate the inhibition zone against some other common bacterial strains.
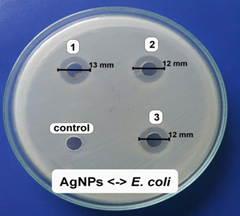
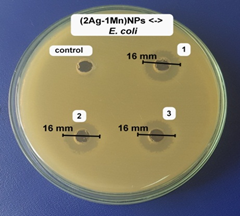
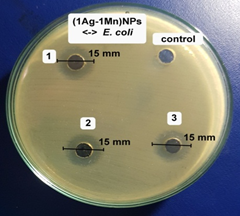
Figure 6. Images of inhibition zone of the samples against E. coli
The antibacterial activity of AgNPs and (2Ag-1Mn)NPs samples against E.coli was clearly shown in Fig.7. Indeed, not only the (2Ag-1Mn)NPs sample had a higher inhibition zone as mentioned above, but also the minimum inhibitory concentration (MIC) against E. coli was much lower than that of AgNPs (4.73 μg.mL-1 compared to 9.45 μg.mL-1).
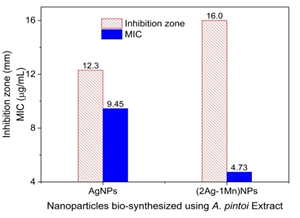
Figure 7. Images of inhibition zone and minimum inhibitory concentration (MIC) of AgNPs and
(2Ag-1Mn)NPs samples against E. coli
The antibacterial activities of the (2Ag-1Mn)NPs sample were further determined against other bacteria strain (shown in Fig.8). The results showed high antibacterial activity against both gram (-) and gram (+) bacteria. The potent antibacterial properties of (2Ag-1Mn)NPs may be attributed to the released metal ions, which could have interaction with microorganisms by means of their attaching to the surface of the cell membranes of bacteria and penetrating into the bacterial cells. The zone of clearance observed against Salmonella, P. aeruginosa, S. aureus, and B. cereus was determined to be 17.7, 19.7, 14.0 and 16.0 mm, respectively.
The MIC values of (2Ag-1Mn)NPs against Salmonella, P. aeruginosa, S. aureus, and B. cereus are determined to be 4.73 mg.mL-1 similar to E. coli. Meanwhile, those for Salmonella and P. aeruginosa are only 2.37 mg.mL-1. The above results show that gram (+) bacteria are less susceptible to Ag+ than gram (-) bacteria. According to Dibrov [20], the NPs are positively charged, so they are convenient to attach to the negatively charged bacteria by electrostatic attraction in the cell membrane. In addition, the gram (+) bacterial cell wall was more peptidoglycan than that of gram (-) bacteria. As peptidoglycan is negatively charged and silver ions are positively charged, as the cell wall of gram (+) is thicker; a large number of silver nanoparticles may get stuck by peptidoglycan in gram (+) bacteria than in gram (-) bacteria [21]. Meanwhile, in gram (-) bacteria, the cell wall has a thinner peptidoglycan layer so the nanoparticles have a better antibacterial effect [22]. Ibrahim's work [23] also showed similar results for gram (-) and gram (+) bacteria strains.
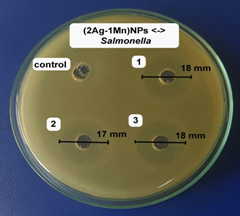
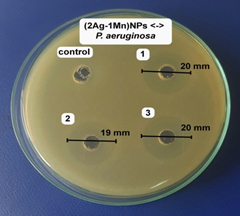
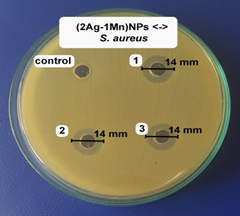
Figure 8. Images of inhibition zone of (2Ag-1Mn)NPs sample against Salmonella, P. aeruginosa, S. aureus, and B. cereus
CONCLUSIONS
The utilization of A.pintoi extract for the synthesis of monometallic and bimetallic nanoparticles under direct sunlight at room temperature proved to be efficient, rapid and environment-friendly. The characterized techniques of nanoparticles demonstrated the formation of highly crystalline particles possessing a spherical shape. The flavonoids, phenolic acids, and alkaloid groups from A.pintoi extract played a vital role in the reduction of Ag and Mn ions to bimetallic NPs, as well as the stabilization of the formed NPs. The results provide evidence that the bimetallic nanoparticles showed better antibacterial activity than the monometallic AgNPs, in which, the (2Ag-1Mn)NPs sample was the most outstanding. In this sample, the zone of clearance observed against E. coli, Salmonella, P. aeruginosa, S. aureus, and B. cereus was determined at the range of 14.0-19.7 mm. Hence, the properties of silver-manganese nanoparticles obtained from A. pintoi extract can unfasten new pathways in the development of new antibacterial agents derived from plant drugs.
REFERENCES