



|
Cardioprotective Effect of Moringa Oleifera Against Doxorubicin Cardiotoxicity in Leukemia Rat Model
Nadia Noble-Daoud Aniss1, Yasser H. Abdel Rahman2, Asmaa M. Zaazaa1* |
|
1 Zoology Department - Faculty of Women for Arts, Science and Education. Ain Shams University, 1 Asmaa Fahmy Street Heliopolis, Cairo, Egypt. 2 Assistant Professor of Cardiology, Cardiology Department, Faculty of Medicine, Benha University. |
ABSTRACT
Background: Acute amyloid leukemia (AML), an aggressive form of leukemia has been majorly treated by the anthracycline anticancer drug Doxorubicin (DOX). Yet DOX has been recorded to be cardiotoxic. Moringa oleifera (MO), a medicinal plant, has been presently exploited to test its anti-oxidative stress, anti-inflammatory, anti-apoptotic potency effect on DOX cardiotoxicity. Objective: the aim of this study was the assessment of any protective effect of MO on cardiotoxicity induced by DOX. Materials and Methods: 70 young adult male albino rats (150 – 180 gm) were allotted into seven groups (10 rats/group): normal control (given i.p. injection with normal saline); MO group (given MO extract 680 mg/day orally); DOX group (given by i.p. injection at a dose of 10mg/kg b.w.); benzene induced leukemia group BIL (induced by intravenous injection of 0.2 mL of a 1:10 diluted benzene solution every 2 days for 3 consecutive weeks); BIL+DOX group; BIL+ MO group and BIL+DOX+MO group for 4 weeks experimental duration. Results: Present results indicate a significant increase in cardiac enzyme levels CRP, CK, and LDH; a significant increase in MDA with a reduction in GSH and GPx oxidative status; a significant increase in TNF-α, NF-ΚB and MCP-1 inflammatory markers; increase in P53, caspase 3 with a decrease in Bcl2 apoptotic markers and a significant increase in cardiac ɤ-H2AX and ET-1 genes in DOX, BIL and BIL+DOX groups. All levels were ameliorated with MO therapy especially noted with the double stress of DOX+MO. Similarly, histological alterations of myocardial vacuolation, fragmentation, nuclear damage, and loss of striation after DOX, BIL, and BIL+DOX treatment were recovered following MO or DOX+MO therapy to BIL rats. Accordingly, MO has shown to be cardioprotective. Conclusion: Concurrent study suggests that MO may be considered as a potentially useful candidate in combination with Dox to limit free radical-mediated heart injury.
Key Words: Moringa oleifera, doxorubicin, cardiotoxicity, leukemia, rats.
INTRODUCTION
Cancer constitutes a major threat to humans and is the second-largest common disease spread worldwide [1]. Leukemias are a group of disorders affecting hemopoietic stem cells by the accumulation of malignant white cells in the bone marrow and peripheral blood. It results from mutations in the DNA that activates oncogenes or deactivates tumor suppressor genes causing disorder of regulation of cell death, differentiation, or division [1].
Benzene, presently used for the induction of acute myeloid leukemia (AML), is an environmental pollutant and occupational toxicant causing various human and animal blood disorders [2]. In addition to inducing hematotoxicity in humans, recent studies have stated hematological effects at several benzene levels including those <1 p.p.m. in the air [3]. This includes acute myeloid leukemia, acute and chronic lymphocytic leukemia, non-Hodgkin's lymphoma, multiple myeloma, and aplastic anemia [4].
Chemotherapeutic approaches are widely applied to overcome the complexities of AML. Doxorubicin (DOX) is an anthracycline anticancer drug effective and frequently used as a chemotherapeutic agent for various malignancies [5]. It is one of the most potent antineoplastic agents used in the treatment of lymphoid malignancies and solid tumors in both adults and children [6]. Doxorubicin hydrochloride (DH) is most often used in the treatment of acute myeloid leukemia (AML) yet induces cytotoxicity. This includes the exact intercalation of planar anthracycline nucleus of DH to the DNA double helix resulting in the prevention of further DNA replication [7].
Nevertheless, cardiotoxicity is the main adverse effect of DOX and other anthracyclines. The risk of some cardiovascular diseases including cardiac dysfunction, dilated cardiomyopathy, hypotension, tachycardia, arrhythmia, and heart failure in cancer limits cancer treatment by DOX [8]. Numerous studies show that various mechanisms such as oxidative stress, inflammation, and apoptotic cell death of cardiomyocytes are involved and lead to the progression of cardiomyopathy after cumulative doses of DOX [9].
Cardiac enzyme biomarkers as C-reactive protein (CRP), creatinine kinase (CK), and lactate dehydrogenase (LDH) are released into the circulation when myocardial infarction or damage to myocytes occurs increasing their levels in the bloodstream thus used as prognostic indicators for major cardiovascular disorders. CRP made by the liver, increases in inflammatory conditions leading to major adverse CVD [10]. CK adds a phosphate group to creatinine to produce high energy phosphocreatine, where its increase could be indicative of heart muscle damage [11]. The activity of lactate dehydrogenase (LDH), an enzyme widely expressed in tissues increases after about 18 hours post myocardial infarction and remains elevated for about a week [10].
Reactive oxygen species (ROS) such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) that detoxify free radicals, are produced under oxidative stress disorders induced by DOX [12]. As the heart lacks the antioxidant enzymes for scavenging free radicals, thus they may accumulate and cause peroxidation of lipids and cardiomyocytes apoptosis [13], thus malondialdehyde (MDA) as a marker of lipid peroxidation increases and antioxidant enzymes, SOD and GSH decrease [12].
Inflammation plays a crucial role in both the initiation and progression of cardiovascular diseases, where several immune cells and chemokines are involved in the inflammatory pathway [14]. Tumor necrosis factor (TNF) in particular induces reactive oxygen species formation, cardiomyocyte hypertrophy, and apoptosis [15]. Also, MCP-1 is a chemokine responsible for the recruitment of monocytes to sites of inflammation [14]. Nuclear factor kappa B (NF-κB) is a protein transcription factor considered as a regulator of innate immunity [16] that is activated upon inflammation where NF-κB dimers are released and translocate to the nucleus [17].
Excessive or insufficient apoptosis, a form of programmed cell death, results in many diseases, including cancer [18] and acute myocardial infarction. Apoptotic markers include Caspase-3, Bcl2, and P53. Overexpression of B-cell lymphoma 2 gene (Bcl-2) significantly reduces infarct size in cardiac diseases through the reduction of cardiomyocyte apoptosis. Similarly, overexpression of cardiac-specific caspase-3 results in increased infarct size [18]. Also, the formation of reactive oxygen species induces p53, and activated p53 promotes apoptosis of cardiomyocytes.
Gamma γ-H2AX is the phosphorylated form of the Gamma H2A histone family member X which forms when double-strand breaks appear. Thus, it is reported that γ-H2AX is a marker that can determine the number of double-strand breaks (DSBs) [19]. ROS oxidize DNA bases and induce DSBs [20]. Endothelin-1 is a powerful vasoconstrictor peptide isolated from endothelial cells. Its manufacture is stimulated during the development of cardiovascular disease. Thus, measurements of endothelin-1 might offer insight into subclinical stages of coronary heart disease [21].
As leukemia is highly aggressive and resistant to chemotherapy, thus several attempts have been made to include natural product therapies as an alternative medicine to attenuate side effects and induce effectiveness. Mainly phytomedicinal plants have been given much emphasis due to their potency and limited side effects. The use of medicinal plants e.g. Moringa oleifera (MO) is beneficial as its bioactive constituents have an impact on multiple biological signaling pathways [22]. It has several natural antioxidant compounds e.g. flavonoids, ascorbic acid, carotenoids, and phenolics, and several parts such as leaves, roots, seed, bark, fruit, flowers, and immature pods are supposed to act as cardiac and circulatory stimulants, possess antitumor, antipyretic, antiepileptic, anti-inflammatory, anti-ulcer, antispasmodic, diuretic, antihypertensive, cholesterol-lowering, antioxidant, antidiabetic, hepatoprotective, antibacterial, and antifungal activities [22, 23]. It can act in synergism with therapeutic drugs to produce an ameliorated therapeutic outcome.
Accordingly, DOX as the highly recommended chemotherapeutic drug for AML treatment, unfortunately, induces cardiotoxicity especially with the longer duration of therapy. Thus, an extra baseline of therapy required for the reduction of doxorubicin-induced cumulative cardiomyopathy and attenuation of the required therapeutic duration represents a major goal in improving its clinical application. The present study is a trial to emphasize on supplementation with a medicinal herb to confront this dilemma. The selected drug presently examined was MO extract due to its potent anti-inflammatory, antioxidant, and anti-apoptotic nature.
MATERIAL AND METHODS
Animals
70 young adult male albino rats (150 – 180 gm. wt.; 6 – 8 weeks of age) were purchased from Theodor Bilharz Research Institute, Cairo, Egypt. Animal procedures were performed in accordance with the declaration of Helsinki and the guidelines for the care and use of experimental animals established by the Committee for Control and Supervision of Experiments on Animals (CPCSEA) and the National Institutes of Health (NIH) protocol. Following housing in metabolic cages, rats were allowed a one-week pre-experimentation adaptation period (food and water ad libitum with daily fresh supplies).
Treatments and Dosage
a. Leukemia Induction: through intravenous injection of 0.2 mL of a 1:10 diluted benzene solution (Chromasolv, in water/2-propanol [50/50] v/v), every 2 days for 3 consecutive weeks [24].
b. Doxorubicin Hydrochloride (DOX) (Sigma Chemical Co., St. Louis, MO): through intraperitoneal injection 10 mg/kg b.w. every other day for four weeks [25].
c. Moringa Oleifera (MO): plant leaves picked from trees at El- Sharkia governorate, Egypt were washed, dried for three weeks then minced into a coarse form. These were macerated in absolute ethanol for 48 hours, then filtered. Ethanol extract was then concentrated and evaporated to dryness at a temperature between 40 and 45°C to avoid denaturation of active ingredients. The concentrated extract was diluted using polysaccharide as a carrier and stored in the refrigerator. MO extract was administered at a dose of 680 mg/day for four weeks [26].
Animal Grouping: Rats were allotted into two main groups; control given i.p. normal saline and AML group given i.v. 0.2 ml benzene. Following three weeks post induction, animals were further allocated into 4 subgroups as follows:
Sample collection: After 4-week experimental duration, blood samples from the retro-orbital venous plexus were collected, dried, and left to clot to obtain sera. Centrifugation at 1800Xg for 10 min. at 4°C followed where aliquots of serum samples were stored at −20°C. Animals were then sacrificed by cervical dislocation and hearts were carefully dissected and washed in saline. The portion of the heart was immediately homogenized to give 10% (w/v) homogenate in the ice-cold medium of phosphate buffer (pH: 7.4) that was further centrifuged at 1800 ×g for 10 minutes at 4°C. The supernatant (10%) was separated and stored at −20°C for biochemical investigations. A second portion was used for the determination of ɤ-H2AX and ET-1 levels using a quantitative real time-polymerase chain reaction (qRT-PCR). A third portion was fixed in 10% formalin saline for histopathological investigation.
Biochemical determinations
Serum C-reactive protein (CRP) was determined by CRP-HS II LT (Latex Turbidimetric Immunoassay) kit purchased from Wako Chemicals GmbH, according to Whicher [27]. Serum creatine kinase (CK) was determined by a kit purchased from Diagnosticum Zrt., according to Mathieu et al. [28]. Serum lactate dehydrogenase (LDH) was assayed by a colorimetric method using kits purchased from BioSystems Co. (Egypt) according to the methods of Young [29]. The cardiac total protein level was determined by the colorimetric method of Lowry et al. [30]. Cardiac malondialdehyde (MDA), glutathione reduced (GSH), and glutathione peroxidase (GPx) contents were determined by colorimetric methods using a Bio diagnostic kit (Egypt) following the methods of Satoh [31], respectively. Cardiac tumor necrosis factor-α (TNF-α) level was determined using the ELISA technique using the human TNF-α ELISA kit purchased from Orgenium Co., Vantaa FINLAND according to Intiso et al. [32]. Cardiac nuclear factor kappa B (NF-κB) level in heart tissue was determined by the ELISA technique using rat nuclear factor kappa B ELISA kit purchased from Glory Science Co., Ltd, USA, according to manufacturer's instruction. Cardiac monocyte chemoattractant protein-1 MCP-1 was estimated by ELISA procedure by using ELISA kit for quantitative detection of rat MCP-1 purchased from Bender MedSystems Co., Vienna, Austria, Europe according to the method of Baggiolini et al. [33]. Cardiac P53 was assayed by the ELISA technique according to the manufacturer's instruction of ELISA kit of rat p53 purchased from Glory Science Co., Ltd, USA. Cardiac caspase3 was estimated by the ELISA technique according to the manufacturer's instruction of ELISA kit for rat caspase 3 purchased from Glory Science Co., Ltd, USA. Cardiac B-cell lymphoma 2 (Bcl2) determined by using the ELISA technique using a kit purchased from Glory Science Co., Ltd, USA, according to the manufacturer's instruction.
Molecular study
Quantitative real time-polymerase chain reaction (qRT-PCR) for determination of ɤ-H2AX and ET-1 expression levels.
Total RNA extraction: Total RNA was extracted from heart samples using SV Total RNA Isolation System (Promega, Madison, WI, USA) according to manufacturer’s instruction. The RNA concentrations and purity were measured with an ultraviolet spectrophotometer.
Complementary DNA (cDNA) synthesis: The cDNA was synthesized from 1 μg RNA using SuperScript III First-Strand Synthesis System as described in the manufacturer’s protocol (#K1621, Fermentas, Waltham, MA, USA). In brief, 1 μg of total RNA was mixed with 50 μM oligo (dT) 20, 50 ng/μL random primers, and 10 mM dNTP mix in a total volume of 10 μL. The mixture was incubated at 56°C for 5 min, and then placed on ice for 3 min. The reverse transcriptase master mix containing 2 μL of 10× RT buffer, 4 μL of 25 mM MgCl2, 2 μL of 0.1 M DTT, and 1 μL of SuperScript® III RT (200 U/μL) was added to the mixture and was incubated at 25°C for 10 min followed by 50 min at 50 °C.
Real-time quantitative PCR amplification and analysis: IT was performed using an Applied Biosystem with software version 3.1 (StepOne™, USA). The reaction contained SYBR Green Master Mix (Applied Biosystems), gene-specific primer pairs which are shown in table (1) and designed with Gene Runner Software (Hasting Software, Inc., Hasting, NY) from RNA sequences from the gene bank. All primer sets had a calculated annealing temperature of 60°C. Quantitative RT-PCR was performed in a 25μl reaction volume consisting of 2X SYBR Green PCR Master Mix (Applied Biosystems), 900 nM of each primer, and 2μl of cDNA. Amplification conditions were: 2 min at 50°C, 10 min at 95°C and 40 cycles of denaturation for 15s and annealing/extension at 60°C for 10 min. Data from real-time assays were calculated using the v1·7 sequence detection software from PE Biosystems (Foster City, CA). Relative expression of studied gene mRNA was calculated using the comparative Ct method. All values were normalized to GAPDH, which was used as the control housekeeping gene and reported as fold change over background levels detected in the diseased groups.
Table 1: Primer sequences used for RT-PCR
|
Gene |
Forward primer |
Reverse primer |
|
Beta-actin |
5'-TGTTGTCCCTGTATGCCTCT-3' |
3'-TAATGTCACGCACGATTTCC-5' |
|
ɤ-H2AX |
5’- GGGCCTAGCTATCCCTCTCCCT 3’ |
5’-CTGCAAAAGTTCCAGTTCAGAAGCCAGA3’ |
|
Endothelin-1 |
5’-TCTCGGAGAG CAGAGACACA-3’ |
5’-TGGACTTTGGAG TTTCTCCCT-3’ |
Histological Studies
10% buffered formalin-fixed cardiac specimens were subsequently processed, sectioned at 6 microns thick, and stained routinely in Hx and E for general histological analysis.
Statistical Analysis
Presently, results were expressed as mean ± standard error of the mean. The significant difference between groups was calculated via the Statistical Package for the Social Sciences program, version 19.0. The difference was considered statistically significant when p<0.05. Percentage difference representing the percent of the variation with respect to the corresponding control group was calculated according to the following formula:
% Difference = (Treated value − control value)/control value) × 100
RESULTS
Biochemical investigations
1- Cardiac Profile
Present results signify a marked increase (P< 0.05) in levels of cardiac CRP, CK, and LDH enzymes in DOX and BIL groups (Table 2). Besides, the treatment of the BIL group with DOX alone induced a higher significant increase compared to the BIL group. Ameliorated measures were recorded following MO therapy to the BIL group being more pronounced with the double treatment with MO and DOX than either alone.
Table 2. Effect of MO or/and DOX therapy on cardiac enzyme levels in control and BIL rats. (Data represented as Mean ± S.E of 10 rats/group).
|
Parameters Groups |
CRP (IU/L) |
CK (IU/L) |
LDH (IU/L) |
|
Con |
2.61±0.41 |
9.22±1.24 |
95.32±10.26 |
|
MO |
2.81±0.52 |
10.03±1.42 |
105.02±11.20 |
|
DOX |
5.24±0.81a |
20.31±3.21a |
136.41±15.42 a |
|
BIL |
6.52±1.01a |
26.62±3.94 a |
176.33±13.71 a |
|
BIL+DOX |
9.32±1.32ab |
38.13±4.62 ab |
210.91±10.82 ab |
|
BIL+MO |
8.03±1.11ab |
33.42±4.21 ab |
194.54±12.92 ab |
|
BIL+DOX+MO |
4.31±0.72abc |
14.76±2.62 abc |
120.62±9.65 abc |
a: Significant change at P< 0.05 compared to the normal control group; b: Significant change at P< 0.05 contra BIL group; c: Significant change at P< 0.05 against the BIL+DOX group.
Results for oxidative and antioxidant status (Table 3) revealed significant elevation (P< 0.05) in the cardiac level of MDA associated with a significant reduction (P< 0.05) in the levels of GSH and GPx in DOX and BIL groups as compared to the normal control group. Similar results were observed as BIL rats were treated with DOX compared to the BIL group. In contrast, treatment of the BIL group with MO or DOX and MO led to a significant reduction in cardiac MDA level associated with significant increment in the contents of cardiac GSH and GPx as compared to the untreated BIL group being more effective in BIL+DOX+MO group.
Table 3. Effect of MO or/and DOX therapy on oxidative and antioxidant status in control and BIL rats. (Data are represented as Mean ± S.E of 10 rats/group).
|
Parameters Groups |
MDA (nmol/mg protein) |
GSH (U/gm tissue) |
GPx (U/gm tissue) |
|
Con |
62.31±6.32 |
26.33±4.14 |
246.62±19.62 |
|
MO |
68.51±8.12 |
24.12±5.12 |
235.20±16.33 |
|
DOX |
105.62±10.51 a |
18.25±4.22 a |
172.13±12.81 a |
|
BIL |
175.82±12.72 a |
12.21±3.82 a |
156.81±13.42 a |
|
BIL+DOX |
240.62±16.20 ab |
8.72±1.61 ab |
126.66±11.31 ab |
|
BIL+MO |
231.87±15.91 ab |
11.51±2.51 ab |
135.42±14.03 ab |
|
BIL+DOX+MO |
145.35±13.58 abc |
21.62±4.11 abc |
206.81±18.75 abc |
a: Significant change at P< 0.05 compared to the normal control group; b: Significant change at P< 0.05 contra BIL group; c: Significant change at P< 0.05 against the BIL+DOX group.
According to Table (4), DOX and BIL groups showed significant elevation (P< 0.05) in cardiac levels of TNF-α, NF-ΚB, and MCP-1 as compared to the normal controls. Nevertheless, more augmented results were shown as the BIL group was treated with DOX compared to the BIL group. In contrast, treatment of the BIL group with MO alone or with DOX reduced cardiac levels of TNF-α, NF-ΚB, and MCP-1 as compared to the BIL group especially noted with double therapy.
Table 4. Effect of MO or/and DOX therapy on inflammatory markers in control and BIL rats. (Data are represented as Mean ± S.E of 10 rats/group.
|
Parameters Groups |
TNF-α (pg/mg protein) |
NF-κB (ng/mg protein) |
MCP-1 (pg/mg protein) |
|
Con |
272.35±11.02 |
85.63±8.21 |
76.21±8.62 |
|
MO |
281.03±16.51 |
89.94±10.63 |
79.22±7.33 |
|
DOX |
352.41±20.21a |
153.41±12.83 a |
103.43±10.41 a |
|
BIL |
405.65±23.61 a |
212.13±9.62 a |
167.34±11.63 a |
|
BIL+DOX |
435.32±15.42 ab |
239.52±15.11 ab |
186.82±9.44 ab |
|
BIL+MO |
421.42±12.81 ab |
227.61±13.44 ab |
175.91±11.92 ab |
|
BIL+DOX+MO |
307.36±14.94 abc |
105.71±8.61 abc |
91.42±8.02 abc |
a: Significant change at P< 0.05 compared to the normal control group; b: Significant change at P< 0.05 contra BIL group; c: Significant change at P< 0.05 against the BIL+DOX group.
Data for apoptotic and anti-apoptotic markers in Dox and BIL group signified significant increase (P< 0.05) in P53 and caspase 3 levels associated with a significant reduction (P< 0.05) in Bcl2 levels compared to the negative control group. A similar increase in P53 and caspase 3 levels and a decrease in Bcl2 were evident in BIL rats treated with DOX recording higher figures. On the other hand, treatment of the BIL group with either MO or both DOX and MO resulted in a significant decrease in P53 and caspase 3 levels accompanied by a significant increase in Bcl2 level as compared to the BIL group with better profiles in the latter.
Table 5. Effect of MO or/and DOX therapy on apoptotic and anti-apoptotic markers in control and BIL rats. (Data are represented as Mean ± S.E of 10 rats/group).
|
Parameters Groups |
P53 (pg/mg protein) |
Caspase 3 (ng/mg protein) |
Bcl2 (ng/mg protein) |
|
Con |
73.52±9.52 |
6.71±0.62 |
4.72±0.41 |
|
MO |
76.12±8.64 |
6.92±0.71 |
4.01±0.35 |
|
DOX |
98.11±9.06 a |
12.42±2.11 a |
2.06±0.18 a |
|
BIL |
135.83±13.22 a |
15.61±2.63 a |
1.82±0.21 a |
|
BIL+DOX |
155.22±12.61 ab |
19.11±3.04 ab |
1.32±0.14 ab |
|
BIL+MO |
138.46±11.03 ab |
13.45±3.62 ab |
1.51±0.16 ab |
|
BIL+DOX+MO |
88.71±7.22 abc |
8.63±1.13 abc |
3.93±0.28 abc |
a: Significant change at P< 0.05 compared to the normal control group; b: Significant change at P< 0.05 contra BIL group; c: Significant change at P< 0.05 against the BIL+DOX group.
Molecular genetics markers
Presently, Dox and BIL treated rats and BIL rats displayed significant amplification (P< 0.05) in the expression of cardiac ɤ-H2AX and ET-1 genes relative to the negative controls (Fig. 1 and Table 6). This was paralleled by significant amplification (P< 0.05) in the expression of cardiac ɤ-H2AX and ET-1 genes in the BIL group treated with DOX alone relative to the BIL group. On the other hand, treatment of the BIL group with either MO or both of DOX and MO led to significant suppression (P< 0.05) in the expression of ɤ-H2AX and ET-1 genes relative to the untreated BIL group. This was more pronounced with the synergetic treatment with DOX and MO to BIL rats compared to the BIL group treated with DOX alone.
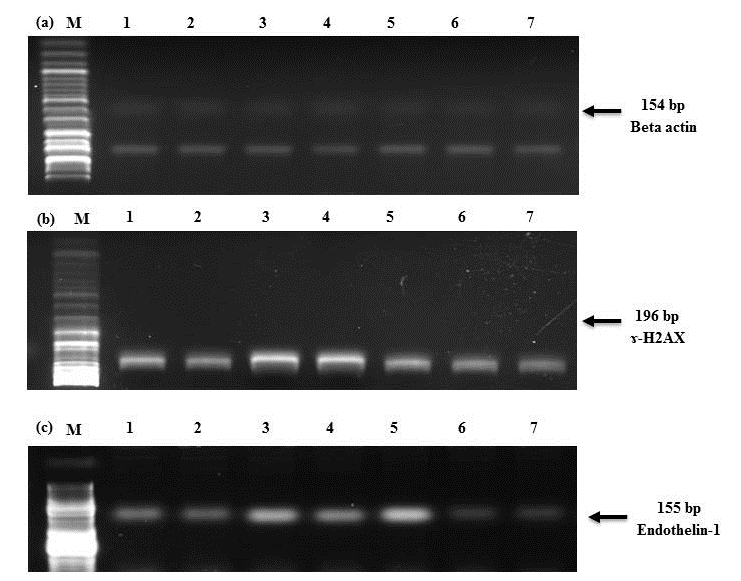
Figure 1(a-c). Agarose gel electrophoresis showing (a) Beta-actin, (b) ɤ-H2AX, and (c) Endothelin-1 mRNA expression in the heart tissue by RT-PCR analysis.
Lane 1: Control group, Lane 2: MO group, Lane 3: DOX group, Lane 4: BIL group, Lane 5: BIL+DOX, Lane 6: BIL+MO, Lane 7: BIL+DOX+MO group. M: DNA ladder (100 bp).
Table 6. Effect of MO or/and DOX therapy on ɤ-H2AX and Endothelin-1 expressions in control and BIL rats. (Data are represented as Mean ± S.E of 10 rats/group).
|
Parameters Groups |
ɤ-H2AX expression |
Endothelin-1 expression |
|
Con |
1.42±0.21 |
1.71±0.16 |
|
MO |
1.53±0.27 |
1.81±0.20 |
|
DOX |
4.62±0.52 a |
4.51±0.41 a |
|
BIL |
6.33±0.71 a |
5.72±0.52 a |
|
BIL+DOX |
7.62±0.80 ab |
8.02±0.92 ab |
|
BIL+MO |
2.91±0.32 ab |
3.2±0.28 ab |
|
BIL+DOX+MO |
2.01±0.24 abc |
2.6±0.23 abc |
a: Significant change at P< 0.05 compared to the normal control group; b: Significant change at P< 0.05 contra BIL group; c: Significant change at P< 0.05 against the BIL+DOX group.
Histological studies:
No signs of heart pathology were found in heart sections of control rats (Figure 2). Rats of MO groups showed well-arranged myocardial fibers in regular rows, the myocardial nuclei were clear, and the myocardial gap was normal (Figure 3) comparing to the DOX group, in which inflammation and fibrosis were observed (Figure 4). Heart tissue of BIL group rats showed myocardial injury represented by myocardial large vacuolation, fragmentation, and lysis, which appeared to be focal (Figure 5a) and was associated with myocardial fibers atrophy and separation from each other. Myocardial lesions were associated with irregular distribution of myocytes' nuclei and severe nuclear damage (Figure 5b). The BIL group treated with DOX showed loss of striation with complete necrosis and fragmentation, highly thickened, and elongated arterial walls containing haemolysed blood cells (Figure 6). The BIL group treated by MO showed myocardial damage in the form of limited minute vacuolation and disordered arrangement of myocardial fibers (Figure 7). The combination of both DOX and MO administered to the BIL group showed a marked decrease in myocardial injury compared with the BIL group where minute myolysis was observed and more or less near to normal appearance of cardiac muscle fibers was realized (Figure 8).
|
Figure 2. Histological examination of the heart of normal control group showing normal heart architecture (H&E; X 400). |
Figure 3. Heart section from MO group showing well-arranged myocardial fibers (H&E; X 400).
|
|
Figure 4. Heart section from DOX group showing inflammation and fibrosis of myocardial fibers (H&E; X 400). |
Figure 5a. Histological examination of the heart of the BIL group showing myocardial large vacuolation, fragmentation, and lysis (H&E; X400). |
|
Figure 5b. Histological examination of the heart of BIL group showing myocardial fibers atrophy and separation from each; myocardial lesions associated with irregular distribution of myocytes nuclei and severe nuclear damage (H&E; X400). |
Figure 6. Heart section from the BIL group treated by DOX showing loss of striation with complete necrosis and fragmentation and highly thickened and elongated arterial walls containing haemolysed blood cells (H&E X400). |
|
Figure 7. Heart section from the BIL group treated by MO showing ameliorated myocardial damage as limited minute vacuolation and disordered arrangement of myocardial fibers (H&E; X 400). |
Figure 8. BIL group treated by both DOX and MO showing normal appearance of cardiac muscle fibers (H&E; X 400).
|
DISCUSSION
The anthracycline anticancer drug DOX for AML treatment has been recorded to be cardiotoxic [5]. Thus, efforts have been made to introduce synergistic safer treatment as an attempt to attenuate the cardiotoxicity. In view of present data, CRP, CK, and LDH showed a significant increase in the BIL group as compared to the normal control group. These results are in line with the study of Han et al. [34] who found that acute leukemia patients with normal temperature or high temperatures had significantly higher levels of CRP than the control group. Thus, leukemia could have a potent effect to increase levels of CK, CRP, and LDH [35]. Likewise, similar increases were recorded following treatment of BIL rats with DOX as compared to the BIL group. After the myocardial injury, the activities of CK, CRP, and LDH in the blood are used to indicate the extent of damage in cardiac musculature [36]. The raised serum LDH activity could be attributed to the considerable increase in free radicals and their effects on the cellular membrane producing leakage of LDH from the damaged membranes of cardiomyocytes into the circulation. It has been stated that oxidative stress is the main reason for cardiac function deterioration that include ischemia/reperfusion injury, myocardial infarction, heart failure, and hypertrophy. High levels of ROS induce cell injuries, including necrosis, and apoptosis [37]. Several mechanisms have been suggested to explain the production of free radicals by DOX. It could combine with iron forming Fe3+ – doxorubicin complex leading to inactivation of cytochrome C oxidase and alterations in iron homeostatic processes associated with aconitase -iron regulatory protein - 1 [38]. The reduced activity of cytochrome C, which is an indispensable component of the electron transport chain, facilitates the production of free radicals. In addition, the lessening of DOX to a semiquinone derivative at complex I of the mitochondrial electron transport chain forms free radicals that improve the levels of oxidative stress [39]. ROS induce damage to mitochondria and cell membranes of the heart muscle cells when reacting with lipids, protein, and other cellular constituents [40]. Aggravating the cardiotoxicity of doxorubicin is the extremely active metabolic machinery in the heart [41], and the occurrence of an inherently low level of the antioxidant defense system in cardiomyocytes [42]. On the other hand, the present study demonstrated that treatment of both BIL group and BIL+DOX group with MO caused a significant decrease in the serum levels of CRP, CK, and LDH as compared to BIL group and BIL+DOX group, indicating its protective effect on the heart by decreasing the myocardial damage, thereby limiting the leakage of these enzymes from the myocardium. The cardioprotective property of alkaloid, an indole and vincosamide, isolated from MO leaves, is involved in preventing the disruption of cardiac myofibrils and thereby improving the cardiac contractile function [43].
In the current study, GSH and Gpx values significantly decreased while MDA levels increased in the hearts of BIL rats compared to healthy controls. MDA is the end product of lipid peroxidation due to free radical attack on unsaturated fatty acids in cell membranes [44]. Lipid peroxidation harms cell membrane integrity and affects its functionality, which initiates apoptosis [45]. In AML patients, the high level of white blood cells was definitely correlated with ROS concentration and MDA levels in blood plasma [46]. MDA is the major product of oxidative degradation of unsaturated fatty acids of the membrane and is a consequence of free radicals overproduction. Increased lipid peroxidation product, MDA, levels, observed in the present study, indicates structural and functional damages in cell membranes. GSH, Gpx, and SOD guard the cells from superoxide attack by dismutation of superoxide radicals to oxygen. When GSH, Gpx, and SOD levels are reduced, the highly reactive superoxide radicals can cause a wide range of cell membrane damage, increase the rate of oxidation of lipids, proteins, and DNA and ultimately result in apoptosis [47].
DOX treatment and treatment of the BIL group with DOX resulted in a significant increase in the cardiac level of MDA associated with significant depletion in the levels of GSH and Gpx as compared to the normal control group or BIL group. The structure of doxorubicin makes it prone to form a free radical in the form of a semiquinone, which reacts with molecular oxygen and produces superoxide anions and hydroxyl radicals [48]. It increases cardiomyocyte capability to reactive oxygen species by decreasing enzymatic and non-enzymatic antioxidants such as superoxide dismutase, catalase, and glutathione. These endogenous antioxidant defenses play a crucial role in the detoxification of ROS [49]. The decreased activities of these antioxidants are attributed to their increased utilization for scavenging reactive oxygen species [50]. In the present study, MO therapy to BIL or BIL+DOX rats resulted in a significant rise in SOD and GSH associated with significant depletion in the cardiac levels of MDA, which may be attributed to its potent free radical scavenging and antioxidant properties. Furthermore, moringa leaves act as a respectable source of natural antioxidant due to the presence of several kinds of antioxidant compounds such as ascorbic acid, phenolics, flavonoids, and carotenoids [51]. Polyphenols add to its scavenging activity [52]. Polyphenols have been known to have powerful antioxidant activity in vitro. They prevent lipid peroxidation by acting as chain-breaking peroxyl radical scavengers and can protect LDL from oxidation [53].
Present data for inflammatory response signified a significant increase in cardiac levels of TNF-α, NF-κB, and MCP-1 in DOX; BIL, and BIL+DOX rats as compared to the normal control group. The complex cascade of pro-inflammatory cytokines that produce the clinical symptoms of systemic inflammatory response syndrome ((SIRS) has been well described [54], and cytokines are known to be secreted by AML blast cells contributing to its biological and clinical symptoms [55]. Pro-inflammatory cytokines (e.g., TNF, IL-1, IL-2, and IL-8) released by cell lysis may cause nitric oxide-mediated vasodilation and inflammatory transudates that result in pulmonary edema and hypotension [56]. Additionally, Inflammation and pro-inflammatory cytokines are predictable mechanisms involved in cardiotoxicity that has reliably been exposed to occur in doxorubicin-injected rats [57]. The inflammatory cascade usually initiated subsequent to oxidative stress-mediated activation of NF-κB, a key transcription factor regulating inflammatory processes [58]. The participation of NF-κB in the pathogenesis of cardiotoxicity has been established in numerous studies [57]. On the other hand, treatment of the BIL group or BIL+DOX group with MO resulted in a significant decline in the cardiac levels of TNF-α, NF-κB, and MCP-1 as compared to BIL and BIL+DOX groups. This reduction emphasizes on the potency of MO to act as an anti-inflammatory agent [59]. Moringa oleifera possesses the anti-inflammatory capacity and can inhibit the levels of TNF-α [60]. This may be due to the occurrence of the anti-inflammatory compounds in MO namely 4-[(2′-O-acetyl-α-l-rhamnosyloxy) benzyl] isothiocyanate, 4-[(3′-O-acetyl-α-l-rhamnosyloxy)benzyl] isothiocyanate, and S-methyl-N-{4-[(α-l-rhamnosyloxy) benzyl]} thiocarbamate [61].
In the current study, DOX, BIL, and DOX+BIL rats manifested a significant increase in cardiac levels of P53 and caspase-3 associated with a significant reduction in Bcl2 levels as compared to normal control or BIL group. It was reported that apoptosis mediated benzene-induced hematotoxicity via the activation of Caspase-3 [62]. Anti-apoptotic member Bcl-2 prevented or delayed cell death, while the pro-apoptotic Bax promoted apoptosis [63]. Activated Caspase-3 leads to irreversible apoptosis [64]. Some studies suggested that the presence of Bcl-2 blocked Caspase-3 activation [61].
Furthermore, increased levels of ROS from DOX activate Bax thus promotes apoptosis [65]. In apoptotic machinery, the proteins of the Bcl2 family and caspases are the checkpoints to regulate apoptosis [66]. Bax initiation confirms cell death by creating mitochondrial pore formation, which leads to mitochondrial cytochrome-C release, poly (ADP-ribose) polymerase cleavage, and finally apoptosis. In this situation, Bcl2 plays a vital role in inhibiting apoptosis by protecting mitochondrial structure and function along with the prevention of mitochondrial permeability transition in cardiomyocytes [67]. The gathering of p53 in the myocardium is another critical factor answerable for doxorubicin-induced cardiomyocyte apoptosis [68]. Cell cycle progression and apoptosis are regulated by p53, which possess the ability to trigger proapoptotic Bax protein to activate the intrinsic pathway of apoptosis in doxorubicin-induced cardiomyocytes [69]. The downregulation of p53 expression either by genetic deletion [70] or by chemical inhibition [71] abrogates cardiomyocyte apoptosis and cardiac dysfunction in doxorubicin-induced animal models.
On the other hand, treatment of the BIL group or BIL+DOX group with MO resulted in a significant decline in the cardiac levels of P53 and caspase-3 associated with a significant increase in the cardiac Bcl2 level as compared to BIL or BIL+DOX groups. MO possesses an antiapoptotic function in cardiomyocytes. It acts as a free radical quencher against DOX generated ROS as observed by a decrease in MDA level. Moringa leaves are rich in phytochemicals including chlorogenic acid and quercetin with potent biological activity. Galuppo et al. (2014) [72] showed that glucomoringin isothiocyanate, a member of glucosinolates present in the Moringaceae family members, increases Bcl-2 expression and decreases P53 expression in the mouse model of experimental autoimmune encephalomyelitis. In line with the current study, previous studies have shown that the antiapoptotic function of quercetin occurs through altering Bax/Bcl-2 expression pattern [73].
In view of present data, ɤ-H2AX and ET-1 expressions were significantly increased in the DOX and BIL groups as compared to the normal control group and in the BIL+DOX group as compared to the BIL group. Toxic damage to the vascular endothelium is the subject of growing attention, as tumor cells require functional endothelium for growth and proliferation [74]. Cardiovascular and oncological diseases are often associated with endothelial dysfunction. Consequently, the rising of ET-1 plasma levels in several types of cancer [75], cardiovascular diseases including chronic heart failure, ischemic heart diseases, is a potential consequence of endothelial dysfunction. On the other hand, ET-1 might have a role in the pathogenesis of endothelial dysfunction, since it exerts paracrine and autocrine effects on endothelial cells [76]. Knowles et al. (2005) [77] stated that ET-1 has a direct angiogenic influence on endothelial cells and indirectly stimulates angiogenesis through aggregate the production of pro-angiogenic factors by fibroblasts and tumor cells. ET-1 acts as a potent vasoconstrictor on smooth muscle cells, whereas in endothelial cells, it induces vasodilatation through the production of NO [76]. Additionally, ET-1 stimulates migration, invasiveness, neovascularization, and promotes tumor proliferation [78]. Moreover, ROS are considered vital parts of the mechanism of leukemia and doxorubicin-induced heart failure due to their pathophysiological effects, which contribute to the loss and functional impairment of cardiac muscles [79]. The excessive production of ROS damages DNA in the myocardium and triggers apoptotic cell death of cardiomyocytes. Doxorubicin-induced DNA damage was assessed by protein expression of γ-H2AX, a marker of DNA double-strand breaks in the myocardium [80].
Nevertheless, Mo treatment to BIL or BIL+DOX groups resulted in a significant decline in the cardiac ɤ-H2AX and ET-1 expressions as compared to the BIL group or the BIL+DOX group. This could be attributed to the protective influence of MO against oxidative-mediated injury. Certainly, the ability of MO to counteract oxidative stress can be associated with high antioxidant activity of its leaves, flowers, and seeds because of ascorbic acid, flavonoids, phenolics, carotenoids [81], quercetin, kaempferol [82], phenolic acids [51] isothiocyanates, polyphenols, and rutin present in leaves [83] Quercetin is crucial as it contains phenolic hydroxyl groups with antioxidant activity [84] and strongly inhibits ROS production [85]. Similarly, luteolin exerts a strong antioxidant activity, a protective effect on DNA [86], and free radical scavenging and anti-inflammatory activities [87].
Histopathological findings showed that treatment with Dox produced enormous changes in the myocardium viewing a varying degree of vacuolar changes in cardiac muscle fibers mainly in the form of degeneration of myocardial tissue, vacuolization of the cardiomyocytes, myofibrillar loss and myocardial hypertrophy [88]. Treatment with MO showed limited minute vacuolation and disordered arrangement of myocardial fibers and this result was in accordance with Mahendra et al. [89].
CONCLUSION
Finally, it could be concluded that DOX-induced cardiotoxicity is related to oxidative stress. Anti-proliferative, anti-initiation, and free radical scavenging properties of MO may boost myocardial integrity and attenuate the cardiac toxicity. MO has shown to be cardioprotective, which may be attributed to its potent antioxidant properties. The current study suggests that MO may be considered as a potentially useful candidate in combination with Dox to limit free radical-mediated heart injury.
Conflicts of interest
No conflict of interest.
ACKNOWLEDGMENTS
Authors acknowledge Prof. Dr. Sanaa Mohamed Rifaat Wahba, Prof. of Histology & Histochemistry - Zoology Department, Faculty of Women for Arts, Science and Education, Ain Shams University for her continuous support, guidance and assistance in language editing.
REFERENCES