



|
Antifungal Properties of Rhizomes of Alpinia calcarata Roscoe from Western Ghats, South India Silvy Mathew1*, Cristiane P. Victório2 |
|
1 Assistant Professor, Post Graduate Department of Botany, Vimala College (Autonomous), Thrissur, Kerala, India. 2 Assistant Professor, Post Graduate Environmental Science and Technology, Fundação Centro UniversitárioEstadual da Zona Oeste (UEZO), Rio de Janeiro, RJ, Brazil. |
ABSTRACT
This study investigated the phytochemical constituents of methanolic rhizome extracts of Alpinia calcarata through the preliminary phytochemical screening, HPLC analysis and its antifungal properties. A.calcarata, Zingiberaceae, is an important rhizomatous perennial medicinal herb and native of India. The phytochemical screening on qualitative analysis showed that the rhizomes of A. calcarata are rich in sterols, anthraquinones etc. which are popular phytochemical constituents and HPLC analysis recorded that it contains five major compounds. Still, there is no proper information on the evaluation of rhizomes of A.calcarata for their antifungal activity. So, methanolic extracts of A. calcarata rhizomes were tested against five important species of pathogenic fungi such as Aspergillus flavus (15.0±0.08), A. niger (13.0±0.07), A. fumigatus (13.0±0.10), Rhizopus stolonifer (11.0±0.13) and Candida albicans (14.0±0.05). The test fungi were mainly associated with seed biodeterioration during storage. Extracts of the rhizome of A.calcarata have recorded significant antifungal activity against species tested. Among these five fungal strains, A. flavus recorded high susceptibility (15.0±0.08).
Key Words: Alpinia, Zingiberaceae, antifungal activity, rhizome, HPLC analysis.
INTRODUCTION
Many plants in Indian traditional medicine, particularly Ayurveda, yoga, Unani, Siddha, homoeopathy and naturopathy have the potential to provide pharmacologically active natural products as a source for new antifungal drugs [1, 2]. Fungi are significant destroyers of foodstuffs and grains during storage [3, 4] and the major toxic effects include carcinogenicity, genotoxicity, teratogenicity, nephrotoxicity, hepatotoxicity, reproductive disorders and immune suppression [5]. Fungi are ubiquitous in the environment, and infection due to fungal pathogens has become more frequent [6]. Another screening for antifungal agents was done on medicinal and fruit-bearing plants used against skin diseases by the Brazilian population. The results, evaluated by the diameter of the inhibition zone of fungal growth, indicated that six plants investigated clearly, showed significant activity against three fungi: Candida albicans, Trichophyton rubrum and Cryptococcus neoformans[7]. The genus Alpinia is a very important plant group to sciences since produce many secondary metabolites classes as flavonoids, tannins, terpenoids and alkaloids [8-19].
Here we used A.calcarata, a perennial herb with non- tuberous rootstock. The stem is slender and 0.6-1.2m high. Leaves are lanceolate, acuminate, green and glossy. The flowers are irregular, bisexual and pedunculate [20] and are arranged in dense panicles, 7.5-10cm long, with pubescent rachis and small ovate bracts. The calyx tube is funnel-shaped and 6-8mm long. Corolla segments are 13mm long. Lip is 2.5-3.8cm long, ovate-oblong, sessile, yellow, streaked with purple veins and emarginate. The ovary is densely pubescent with many ovules in each cell. In A.calarata the capsules are globose and red [21]. The whole plant body is used as medicinal (Figure 1), especially the rhizomes are widely used for curing many diseases like bronchitis, asthma and also used to stimulate digestion and used as a purifier of blood and voice. The previous reports show that it contains many biologically important compounds like methyl cinnamate (48%), 1,8-cineole (20-30%), pinene and camphor, nerolidol [22]. Experimental studies have confirmed the presence of antidiabetic activity of this plant [23]. Arambewalaet al. [24] have proved that the presence of 18 volatile compounds in the essential oil of A.calcarata.The rhizomes of A.calcarata are inflammatory [25] seed is anti ulcerative [26] and rhizomes are with antifungal, disinfectant, stimulant activities [27]. Jantanet al.[28] studied the antifungal activity of the terpenoids of essential oils of nine Zingiberaceae species.
Flavonoids are common in most of the Zingiberaceae plants and its first report in A. calcarata in 2009 [29]. A. calcarata is an active natural aphrodisiac in traditional medicine in many parts of the world [30, 31] and possess aphrodisiac activity of [32]. Several studies in A.calcarata revealed that it possesses antibacterial [33], antifungal [34], antihelmintic [35] and antinociceptiveactivities. Further, essential oil of A. calcarata is shown to have repellant activity against the common cockroach Periplanetaamericana [36].
Thus, several studies using rhizome extracts of Alpiniaspp. and essential oils have been carried out from several countries. However, the validity of the antifungal activity of rhizomes of A.calcaratahas not been scientifically investigated so far. Therefore, this study aimed to investigate the antifungal potential of A.calcarata rhizomes extracts.

Figure 1.Organs of Alpinia calcarata plants used as medicinal: A. Leaves, B.Rhizomes and C. Inflorescence.
MATERIALS AND METHODS
Plant Material and Fungi
The fresh rhizomes of Alpinia calcarata were collected from Kumily, Idukki, Kerala and were identified by Dr S John Britto, Director, Rapinat Herbarium and Centre for Molecular Systematics, Tiruchirappalli. Fresh A.calcarata rhizomes were cut into small pieces; air-dried for 12-15 days in the shade and coarsely powdered rhizome of 80g was subjected to successive soxhlet extraction with solvents of increasing polarity petroleum ether, acetone, methanol and water respectively.
Preliminary Phytochemical Screening
Preliminary phytochemical studies were carried out to characterize the therapeutically active constituents according to the procedures and methods outlined [37,38]. The high polarity solvent methanol extracted a higher quantity of secondary metabolites of medicinal importance. So, the methanolic extracts of rhizome were used for phytochemical screening to detect major chemical groups. Thin Layer Chromatography (TLC) was performed on commercial glass plates precoated with 0.25 mm layers of silica gel F254 (Merck). The plates were activated at 1200C for 30 min and then used. On a coated TLC plate the solution of the extracts (5 µL) was applied as a thin spot. Chromatograms were run in small glass tanks linked with chromatography paper equilibrated with the running solvent (hexane:ethyl acetate). When the solvent front reached below the upper edge of the plate it was withdrawn and the solvent was allowed to evaporate. Spots and bands of compounds on TLC were detected using UV light. Also, a spraying reagent (potassium permanganate) was sprayed and the coloured spots formed were noted. The resolution bands were obtained and retention factor (Rf) values were calculated. The movement of solute is expressed by its Rf value.
HPLC Analysis
Methanolic rhizome extract (40mg/mL) of the rhizome of A. calcarata was subjected to High-performance liquid chromatography (HPLC) analysis. The record was superimposed on the retention time values of this extract. HPLC analysis was performed on Shimadzu LC 10 AVP HPLC system equipped with a binary pump SPD 10 AVP pump. A sample was filtered through an ultra cellulose nitrate membrane filter before injection into the sample. 10 μL of the filtered sample was injected to the automatic injector using a microsyringe (1 - 20 μL, Shimadzu). The analysis was done on the LC column with a reverse-phase C-18 - Phenomenex and column dimension of 5 cm x 1.5 cm. The mobile phase was 2% THF in water1% THF in acetonitrile (40:60) in an isocratic method. The sample was eluted for 5 minutes at a flow rate of 2.0 mL/min with a column temperature maintained at 25 ± 20C at 254 nm. The HPLC system was equipped with software class VP series version 6.1(Shimadzu). The chromatogram with specific retention time and peak area of the standard and sample were recorded. The method used for the quantification is the area under the curve method. The equation used for the quantification is as follows:
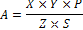
Where,
A = percentage of marker compound in the drug
X = concentration of the standard marker injected
Z = area given by the standard compound
Y = area given by the marker compound in the sample profile
S = sample concentration with respect to the drug taken
P = purity of the marker compound taken
Preparation of Antifungal Medium
The test fungi were maintained in Potato Dextrose Agar (PDA) slants. (HiMedia Laboratories Pvt. Ltd., Mumbai). The potato tubers were peeled off and weighed to about 250 g. The tubers were sliced into small pieces in the sterile conical flask. After boiling, the supernatant was collected and dextrose with agar to dissolve the ingredients. The medium pH was adjusted to 5.6±0.2 at 250C. These constituents were mixed and finally, the medium was sterilized for 20 min and poured into Petri plates for solidification and was used for antifungal studies. The medium without extracts saved as control and each treatment was replicated three times. Plates were incubated in an incubator at 280C till the control reached full growth.
Assay of Antifungal Activity
From the extractives, the methanolic extract showed more antibacterial effect, so, it is used for the antifungal assay. A voucher specimen is deposited at the Rapinat Herbarium and Centre for Molecular Systematics, Tiruchirappalli with under accession number RHT 66467 dated 24th Dec.2012for further reference. The fungal strains were Aspergillusniger MTCC # 2612, Aspergillusflavus MTCC # 2813, Aspergillusfumigatus MTCC # 2584, Candidaalbicans MTCC # 1637 and Rhizopusstolonifer. The effect of active methanolic extract of A. calcarata was investigated for in vitro antifungal activity against five selected fungal strains: A. niger, A. fumigatus, A. flavus, C. albicans and R. stolonifer. The antifungal activities of the methanolic extracts (200µL/L) concentration used for agar well diffusion method [39] (Figure 5). The test fungal culture was evenly spread over the media by sterile cotton swabs. The wells (6mm) were punched over the agar plates using sterile gel puncher and plant extract 200 µL were transferred into the separate wells. The plates were incubated for 24 hours at 370C. After incubation, the diameter of inhibitory zones formed around each disc was measured in mm and recorded. Each experiment has three replicates and three determinations were conducted. Means of variable and standard error (±) were calculated. The fungitoxicity of the extracts in terms of percentage inhibition of mycelial growth was calculated by using the formula
Where dc = Average increase in mycelial growth in control, dt = Average increase in mycelial growth in treatment [40].
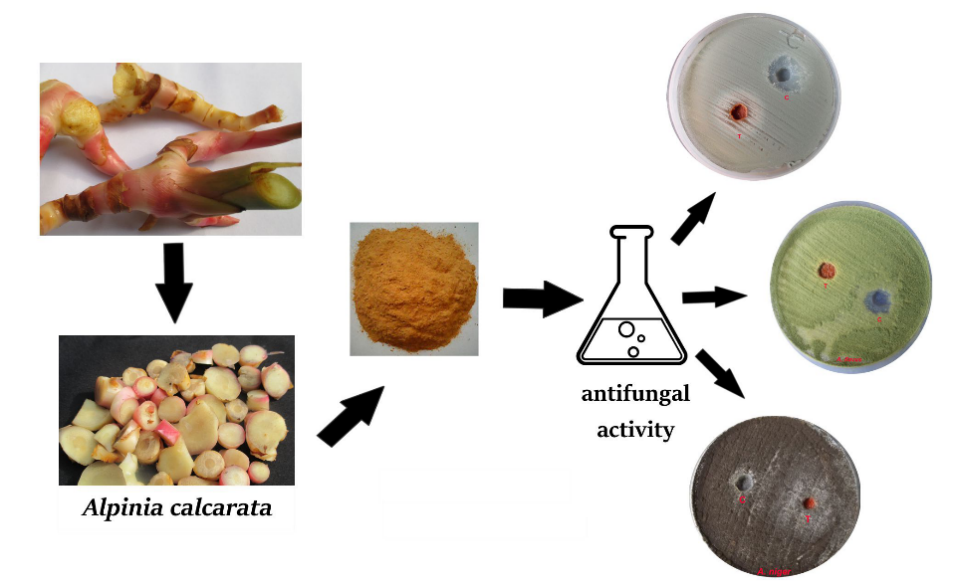
Figure 2. Schematic representation of the antifungal assay using extracts of the rhizome of Alpinia calcarata.
Statistical Analysis
The data were calculated as mean ± SD and analyzed using statistical analysis. We used Ho (mean values are the same) and H1 (mean values are not the same). Each experiment has three replicates. Three determinations were conducted and the significance level for all measurements was considered at 𝑃< 0.05
RESULTS AND DISCUSSION
Phytochemical screening
The curative properties of medicinal plants are perhaps due to the presence of various secondary metabolites such as alkaloids, flavonoids, glycosides, phenols, saponins, sterols etc. The phytochemical screening on qualitative analysis shows that the rhizomes of A. calcarata are rich in sterols, anthraquinones etc. which are popular phytochemical constituents (Table1). TLC profiling of the extract confirmed the presence of various phytochemicals. The Rf values obtained for the major spots are 0.85 and 0.83.
Therefore, the data generated from these experiments have provided the chemical basis for the wide use of this plant as a therapeutic agent for treating various ailments. However, there is a need to further carry out advanced hyphenated spectroscopic studies to elucidate the structure of these compounds. Also, these tests facilitate their quantitative estimation and qualitative separation of pharmacologically active chemical compounds. Furthermore, this data may be handy in probing of the biochemistry of this plant.
Table 1. Preliminary phytochemical screening of methanolic rhizome extracts of Alpinia calcarata.
|
Chemical Classes |
Tests performed |
Status |
|
Carbohydrates |
Molisch’s test |
- - |
|
Phenols |
Phosphomolybdic acid test |
- - + |
|
Flavanoids |
Lead acetate test |
- + + |
|
Terpenoids Sterols |
Salkowski test Salkowski’s test |
+ + + + + + |
|
Alkaloids |
Mayer’s test |
+ + |
|
Anthraquinones |
Borntrager’s test |
+ + |
|
Amino acid test |
Ninhydrin test |
++ |
|
Fixed oils and fats |
Fixed oils and fats test |
- - |
+ + +High amount, + +Moderate quantity, +Traces, - -Absence
HPLC Analysis
Five compounds were detected through the HPLC at 254 nm as shown in Table2 and Figure 3. The most valued secondary metabolite, nerolidol, (sesquiterpenoid) from the rhizome of A. calcarata was in a fairly high concentration (2.745%, w/w).
Table 2.Peak areas of compounds detected by HPLC from methanolic rhizome extract of Alpinia calcarata.
|
Peak |
RT (min) |
Relative area (%) |
|
1 |
0.28 |
166 |
|
2 |
0.39 |
98 |
|
3 |
1.42 |
200 |
|
4 |
1.81 |
74 |
|
5 |
2.50 |
132 |
RT. Retention time
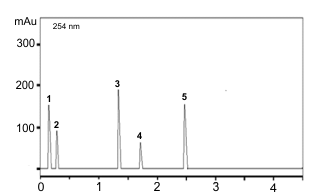
Figure 3. HPLC chromatogram of methanolic rhizome extract of Alpinia calcarata.
Antifungal Assay
Aspergillosis is caused due to inhalation of A. fumigatus spores. It is an opportunistic pathogen which usually affects cavities that have formed in the lungs from pre-existing lung diseases. In the lungs, A. fumigatus forms a tangled mass of fungus fibres and blood clots. The fungus mass gradually enlarges, destroying lung tissue in the process, but usually does not spread to other areas. Also, a significant portion of the agricultural produce in India and the world over become unfit for human consumption due to mycotoxins contamination of grains, especially those produced by species of Aspergillus [41, 42]. Also, C. albicans is responsible for the most common skin disorder.
Our present work was designed to perform the antifungal studies on active methanolic extract of A. calcarata. All the checked fungal strains showed moderate antifungal activities (Table 3, Figure 4). Inhibition zones in A. calcarata methanolic extracts were A. flavus (15.0± 0.08), A. niger(13.0±0.07), A. fumigatus (13.0±0.10), R. stolonifera (11.0± 0.13) and C. albicans (14.0± 0.05).
Table 3.Antifungal assay of Alpinia calcarata against fungal strains.
|
Samples |
Fungal Strain |
ME(C)* |
ACM(T)** |
|
1 |
A.niger |
8.0±0.13 |
13.0±0.07 |
|
2 |
A. fumigatus |
15.0±0.15 |
13.0±0.10 |
|
3 |
A. flavus |
8.0±0.09 |
15.0±0.08 |
|
4 |
C. albicans |
13.0±0.05 |
14.0±0.05 |
|
5 |
R.stolonifer |
12.0±0.08 |
11.0±0.13 |
ME(C)*methanol (control). ACM(T)**- A.calcarata methanolic extract.
Among these five strains, A. flavus showed the highest activity (15.0±0.08). Our present study demonstrates the antifungal potentialities of A. calcarata, which would improve our understanding of the biological role of the plant and a future avenue to develop new antifungal therapies. This study evaluated the inherent antifungal activity of methanolic extract of A. calcarata. In the present study, methanol alone showed 15.0±0.15 activity but at the same time, A. fumigatus showed only 13.0±0.10. From the results obtained it can be concluded that methanol in itself has antifungal activity.
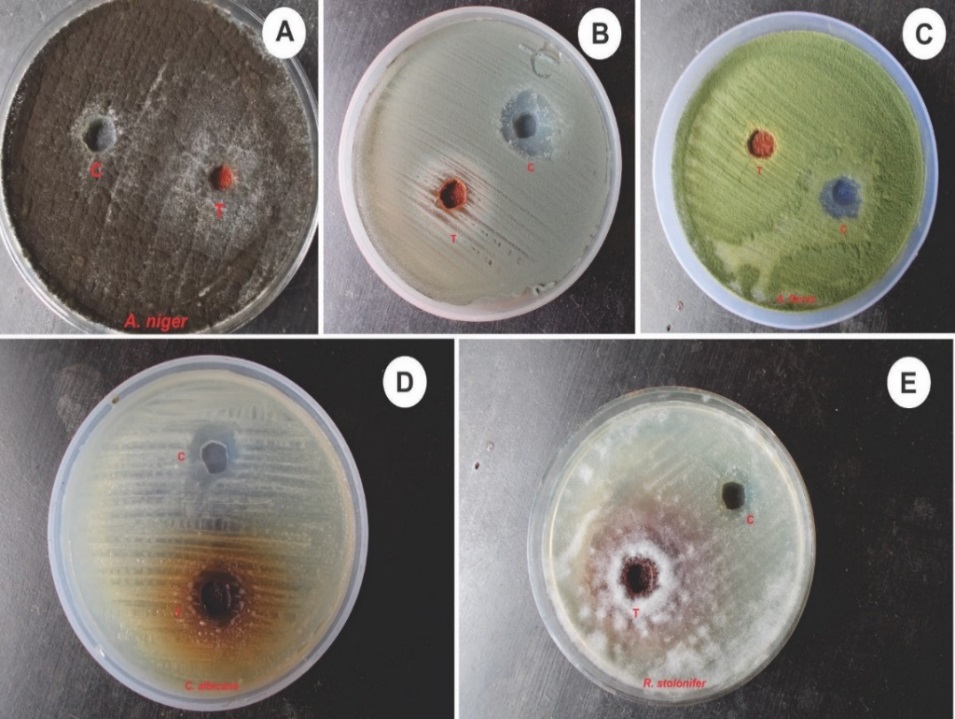
Figure 4. Antifungal assay of methanolic extract of rhizomes of Alpiniacalcarata against -A. Aspergillusniger, B.A.fumigatus, C.A. flavus, D. Candida albicans, E. Rhizopus stolonifer. Inside plate C – control and T - treatment.
It was clear from this study that the solvent extraction and method of extraction affected the degree of antifungal activity. Other factors such as the environmental and climatic conditions of the plants also affected the degree of antifungal activity. Successful prediction of botanical compounds from plant material is largely dependent on the type of solvent used in the extraction procedure. In antifungal studies among the five checked fungus, highest IZ observed in A. flavus with 1±0.8. Findings in this study confirmed that plant extracts can be used as natural fungicides to control pathogen fungi to reduce the dependence on the synthetic fungicides. That means the understanding of the inhibitory mechanism would provide better guidelines toward the development of efficient production and application of technologies associated with bio fungicide plant materials. Also, it is revealed that traditional plants are a valuable source of novel antifungals. From the data, we calculated test statistic is -1.296 and table value is 2.306. So, we can conclude that our value is numerically less than the table value. So we accept H0, that means mean values are the same.
CONCLUSION
In the present study, we have found that five major biologically active phytochemicals were present in the methanolic rhizome extracts of A. calcarata. The phytochemical screening and quantitative estimation by using HPLC showed that rhizomes of A.calcarata are very rich in terpenoids, sterols, flavonoids etc. Since the methanolic extract of rhizome can be considered beneficial for further investigation. The medicinal properties especially the antifungal property of rhizome extracts of A. calcarata may be due to the presence of these phytochemicals. The presence of these phytochemicals indicates that this plant is a good anti-microbial agent.
The results obtained from this work also showed that rhizome extracts of A.calcarata exhibit antifungal effects against A.flavus, A. niger, A. fumigatus, R. stolonifer and C.albicans. Thus it is concluded that A. calcarata rhizomes possess a strong and safe antifungal activity. Nowadays, the production of artificial fertilizers are on the rise in agricultural filed with their harmful effects, so, it is the need of the hour to find out natural ways to reduce the attack of microorganisms like fungi. The results of the present investigation are an important step towards crop safety strategies for antifungal activity against important seed-borne of A. flavus. Thus, we can also conclude that the production of secondary metabolites from medicinal plants will reduce the reproduction of undesirable microorganisms and it carries a prominent role in the production of pesticides and the other crop improvement strategies. Further research will be done for extending commercial formulation based on field trial and toxicological experiment.
ACKNOWLEDGEMENTS
The authors are grateful to DST – FIST Laboratory, Vimala College (Autonomous), Thrissur and Rapinat Herbarium and Centre for Molecular Systematics, Tiruchirappalli for providing institutional facilities. The authors are also thankful to Dr.S John Britto, Taxonomist & Director, Rapinat Herbarium and Centre for Molecular Systematics for the identification of plant species.
Conflicts of Interest
The authors declare that they do not have any conflicts of interest in this research.
REFERENCES