|
Antidiabetics and Glucose Uptake Activity of Prosopis Farcta on STZ-Induced Diabetic Rats and 3T3 L1 Adipocyte Cell Line Fatemeh Nosrati1, 2, Mahboobeh Gholami1, 2, Abolfazl Mohammadi1,3, Najmeh Nazari4, Hamidreza Hemati5, Farshad Zamani1, Hossein Nazari1, Hadi Amrollahi6*
|
|
1 Department of Biochemistry, Semnan University of Medical Sciences, Semnan, Iran. 2 Research Committee Student, Semnan University of Medical Sciences, Semnan, Iran. 3 Department of Neuroscience, Faculty of Advanced Technologies in Medicine, Iran University of Medical Sciences, Tehran, Iran. 4 Department of Biochemistry, Faculty of Basic Science, Azad University, Science and Research Branch, Tehran, Iran. 5Department of Surgery, Semnan University of Medical Sciences, Semnan, Iran. 6Department of Medical Mycology and Parasitology, Semnan University of Medical Sciences, Semnan, Iran. |
ABSTRACT
Introduction and Objectives: Today researchers are trying to find the anti-diabetic effects of dietary and medicinal plants as alternative medicines and chemicals, which have been traditionally used for centuries. The purpose of this study was to investigate the hypoglycemic effects of the extract of Prosopis farcta on adult male rats and glucose uptake activity in 3T3 L1 adipocyte cells. Results: Groups treated with the doses of 40 mg/kg BW of the extract lowered the blood glucose levels similar to glibenclamide. A significant decrease in plasma glucose levels was shown in the rats treated with 60 mg/kg BW of the extract compared with the group treated with glibenclamide. The results of this study indicate that the glucose-lowering property of the Prosopis farcta extract is more effective than glibenclamide. The maximum blood plasma glucose level lowering was shown after 2 days of treatment with the extract of P. farcta at 60 mg/kg BW oral and 8 ml/kg BW subcutaneous administrations compared to the control group (p<0.001). Doses of 800 µg/ml of the aqueous extract of P. farcta had the maximum 2- deoxyglucose uptake in 3T3 L1 adipocyte cells (p<0/001). Conclusion: The results confirmed the hypoglycemic effect of the P. farcta extract and its traditional use in the treatment of diabetes. In addition, findings showed that aqueous extract of P. farcta fruit is effective in increasing the level of glucose uptakes into the adipocytes and this effect is promoted by increasing the extract dose because it is concentration-dependent. The data of this study suggest that the P. farcta fruit may be prescribed as an adjunct to the traditional formulations and drug treatment for controlling and monitoring type 2 diabetes mellitus.
Key Words: Diabetes, Prosopis farcta, Hypoglycemic, Rat, 3T3 L1 adipocyte cells.
INTRODUCTION
Diabetes mellitus is a common disease that is presently 6.6% in the world and over 3 million people in Iran. [1]. It is caused by various factors, including the destruction of insulin-secreting cells in the pancreas and insulin resistance, which cannot send the insulin message into the cells. The most important symptoms of hyperglycemia are polyuria, polydipsia, polyphagia, glycosuria, and a common complication of metabolic acidosis that is dangerous and may lead to shock [2]. There are many plants in natural resources, used in the traditional medicine of different nations to treat diabetes or reduce its complications. The hypoglycemic effect of many of these herbs has been reviewed and approved, but the antidiabetic properties of many of these herbs have not yet been addressed [3]. The long-term use of chemical drugs for the treatment of diabetes and their unwanted side effects have led the attention of medical researchers to the use of herbal remedies, which are natural and have fewer side effects [4]. In addition, some commonly used drugs for the treatment of diabetes mellitus, such as metformin, have herbal bases [5]. The use of medicinal herbs for the treatment of diabetes is widespread in the world, including Iran [6]. However, currently the use of insulin and hypoglycemic agents, are the main and effective treatments for diabetes mellitus, but they have many unpleasant complications, such as increased fat stores, loss of adipose tissue in the injection site, problems during injection and occurrence of hypoglycemic shock, and in the long term, they do not affect the pathogenesis of debilitating complications of diabetes. Due to the increased human knowledge about the heterogeneity of this disease, the need to find effective compounds in the treatment of diabetes is less likely to be associated with side effects. Due to the prevalence of diabetes in today's societies and the high incidence of chemical drugs, there is a need for drugs with minimal complications and high degrees of confidence to be used for a long time [7]. The use of medicinal herbs in the treatment of diabetes has been the subject of many controversial studies in medicine. The history of the first clinical trial in this field is about 70 years ago. Today, the limitations that exist especially in the treatment of chronic diseases, as well as the side effects of synthetic drugs, have caused the attention of doctors over time to focus on the other therapeutic areas which are entitled: “alternative medicine or complementary medicine” [8]. Herbal medicine, as the most common alternative medicine and herb, is an important part of the treatment of diseases. So far, more than 400 plant species worldwide have been recognized as effective agents in the treatment of diabetes. However, many of them have not yet been found to have significant research evidence [9, 10]. For the scientific introduction and application of the antidiabetic effects of a plant, factors should be taken into account including the traditional use of the plant in more than one country, laboratory testing of its effective ingredients, blood glucose-lowering effects, low toxic effects, abundance, affordability, and accessibility [11]. For a long time, Prosopis farcta has been used in the Middle East (Jordan) for the treatment and control of diabetes in traditional medicine [12]. Scientific evidence of the anti-scrotum effects of the plant has also been mentioned in the scientific journals of herbal medicines [13]. However, there is no established scientific evidence regarding its anti-diabetic effect. In addition, diversity in the ecotypic geographic system causes differences of the plant species in different regions, which includes changes in the amount and types of plant effectiveness and second metabolites. The aim of this study is to investigate the antidiabetic effect and glucose uptake activity of P. farcta extract and the introduction of plant for therapeutic use in diabetes.
MATERIALS AND METHODS
Materials
Materials and necessary tools, used in this study are as follow. DMEM High and Low glucose medium for the general growth of the adipocytes and to exposure differentiation and the drugs on them, respectively. Streptozocin (STZ) “# SIG-S0130”, ether alcohol and glibenclamide from Sigma Aldrich, dextrose serum 5%, culture plates, insulin, glucose, glucose uptake measurement kit (Abcam #: ab136955, Version 2015), cytochalasin D, ELISA Reader, inverted microscope (Nikon), glucometer “Glucocard 01, Arkray and a shaker incubator.
Plant collection
The fruits of P. farcta were collected from Semnan province during June-July and identified by Botanical Survey of Iran, Semnan, and were dried under shade, powdered and extracted with hydroalcoholic and water, using a Soxhlet and water steam apparatus respectively. The resulting extract was evaporated on a water bath to give a dry extract and then, it was stored in a refrigerator. The weighed quantity of the dried extract of P. farcta was reconstituted in normal saline and evaluated for pharmacological activities.
STZ-induced diabetes in male Wistar rats
Male Wistar rats (200-250 g) were used for the investigation and were housed in the standard environmental conditions and fed with a standard pellet diet. All the pharmacological study protocols had been met with the approval of the Institutional Animal Ethics Committee. The method, described by Lorke was employed for the determination of the LD50 (14, 15). Animals were observed 24 h after drug administration. The general symptoms and signs of toxicity, water, and food intake, and mortality were evaluated and recorded. The LD50 value was calculated from the square root of the lowest lethal and the highest non-lethal doses products i.e., the geometric mean of the consecutive doses of 0 and 100% survival rates. Forty-eight male Wistar rats were divided into eight groups; each consisted of six males, weighing 200-250 g. They were fasted overnight and administered with the extract at the following doses: 10, 20, 40, and 60 mg/kg BW orally, and 8 ml/kg BW injected subcutaneously (SC). The rats of the second group fasted for 18 h and after that, they were made hyperglycemic by a single intraperitoneal injection of STZ which was dissolved in 3 mM citrate buffer, pH=4.5, at the dose of 50 mg/kg body weight. After 48 h, rats exhibiting plasma glucose level of more than 250–300 mg/dl were chosen for the study and divided into the groups, containing six animals. One group served as control and did not receive any treatments, the second group received the standard drug Glibenclamide (600 µg/kg BW) as the positive control, and the third group served as negative control, which contained diabetic animals, receiving normal saline, and other five groups received hydroalcoholic extract reconstituted in saline (10, 20, 40 and 60 mg/kg BW, orally and 8 ml/kg, SC). Blood glucose was measured through the tail vein of the rats daily before drug administration. The blood sugar concentration was determined by a glucometer and the results were recorded for analysis.
Cell culture
Cells
3T3 L1 adipocyte cells were used for these studies. Cells were used for determining the effect of P. farcta extract on insulin-stimulated glucose uptake by colorimetric assay. 3T3 L1 adipocyte cells were maintained in DMEM medium supplemented with 10% (vol/vol) fetal bovine serum [FBS, ICN Biomedical Inc., Aurora, Ohio) and Gentamycin (100 U/ml). For glucose uptake studies, the cells were treated with trypsin, transferred into a 96 well plate and incubated at 37º C in a 5% CO2 atmosphere. Cells were grown in DMEM medium [DMEM, Sigma, Saint Louis, Missouri] containing 2% FBS (vol/vol) and 0.1% Gentamycin in a 5% CO2 atmosphere at 37ºC and were used in experiments after six days of growth, before reaching the confluence. Cytochalasin D ”as an actin cytoskeleton inhibitor” was obtained from Calbiochem Inc. (San Diego, California).
Colorimetric assay of glucose uptake
1500 cell/well of 3T3 L1 adipocyte cells were cultured in a 96- well plate, containing 100 μL/well of DMEM, with 10% FBS, and the cultured cells were incubated in the appropriate conditions (37 ᵒC and 5% CO2). Differentiated cells obtained after at least four days by using 2% FBS, were divided into 8 groups; control group (without any stimulation), positive control (glucose uptake stimulation with 10 nM insulin), negative control (inhibition of glucose uptake with 1 µM of Cytochalasin D, and two treatment groups (glucose uptake stimulation with aqueous P. farcta extract with the concentrations of 400 and 800 µg/ml). The 2 - Deoxy-glucose, uptaken by cells, was assessed by using the glucose measurement kit instruction (ab136955). The cells in the 96 well plate were incubated in 2 ml Krebs Ringer HEPES [KRH] buffer for 40 min at 37 ᵒC to get severe glucose starvation after washing with the phosphate-buffered solution (PBS). The control group cells were washed for three times with PBS and kept until the cell lysis step, but the treatment group cells were exposed to 10 nM insulin for 20 minutes, Cytochalasin- D (30 minutes, as an actin filament polymerization inhibitor) and the herbal extracts (30 minutes). Cells were stimulated by 10 nM insulin for 20 minutes to activate glucose transporter proteins in cells. 10 μL of 10mM 2-Deoxy Glucose (2-DG) solution was added to the cells and mixed with by pipetting and the cells were incubated for 20 minutes in the appropriate condition. 80μL of the extraction buffer was added to each well for down lysis. The lysed cell solution was heated at 85 ° C for 40 minutes so that the intracellular NADPs were removed from the cells and the enzymes existing in the sample were destroyed. Then the cell lysates were stayed on the ice for 5 minutes and neutralized with 10 μL of Neutralization buffer before transferring the supernatant into new tubes. The reaction was conducted in a flat bottom 96- well plate and it is recommended to dilute samples, 1/10, in the assay buffer to a total volume of 50 μL. According to the kit instructions; 10 μL of Reaction Mix A. (NADPH generation) was added to each well and mixed well by pipetting and then incubated for one hour at 37 ° C. Then 90 μL of the extraction buffer was added to each well. The microplate was sealed with parafilm and heated for 40 minutes at 85 °C to degrade any NADP left in the sample. The microplate was incubated on ice for 5 minutes to be cooled and the reaction was neutralized by adding 12 μL of neutralizing buffer. Then according to the instructions kit, 38 μL of Reaction Mix B was added to each well and mixed well by pipetting. Absorbing the color complex, produced in the wells was recorded at the wavelength of 412 nm using the ELISA microplate reader. Then, the received data were analyzed on the basis of the kit instructions.
Data analysis:
In this study, the data were recorded as Mean ± S.EM and finally, the results were compared with the treatment, control, and standard groups in the respective doses. Statistical difference was tested by using one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls’ comparison test. A difference in the mean p-value <0.05 was considered as statistically significant.
RESULTS
Effect of hydroalcoholic extract of P. farcta with 10 and 20 mg/kg on Blood Glucose Reduction
In this study, the effect of hydroalcoholic extract of P. farcta on blood glucose of rats was evaluated and the results were compared with the control group and glibenclamide as a standard drug. The cells of each group were treated as follow; the treatment groups with the extract of P. farcta with 10, 20, 40, and 60 mg/kg body weight, the positive control group by glibenclamide, and the negative control group by the orally physiologic serum with water. Blood glucose was recorded daily at fixed hours by a glucometer. The results were compared using statistical methods between treatment and control groups. The data analysis indicated that administration of P. farcta extract at the doses of 10 and 20 mg/kg BW in the treatment group did not decrease blood glucose in comparison to the positive control group. (Figures 1 and 2).
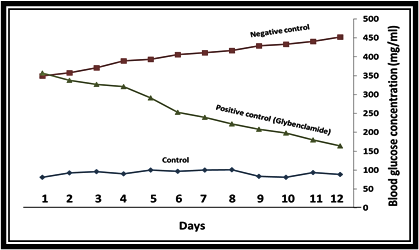
Figure 1. The effect of glibenclamide on plasma glucose levels. A single oral dose of Glibenclamide decreased plasma glucose levels with the maximum effect occurring 5 days after treatment in comparison to the control groups. (P<0.01)
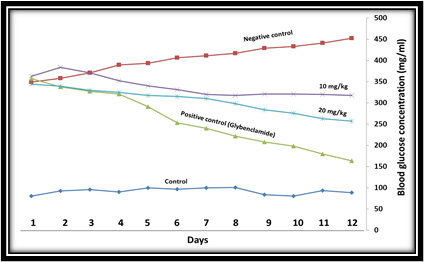
Figure 2. The effect of 10, 20, mg/kg BW of the single oral doses of P. farcta hydroalcoholic extract, on STZ-induced diabetic rats.
Effect of P. farcta extract at the doses of 40 and 60 mg/kg on Blood Glucose reduction
The treatment dose of 40 mg/kg compared to the positive control group showed similar effects and reduced blood glucose levels. In order to confirm the effect of hydroalcoholic extract of the plant on reducing blood glucose, animals were tested at a dose of 60 mg/kg. The results showed that 60 mg/kg body weight significantly decreased the blood glucose levels on the second treatment day in comparison to the positive control group (p <0.001). This indicates that the blood glucose in diabetic animals is reduced in the oral administration of P. farcta, while according to the results of the administration of 20 and 40 mg/kg body weight to the rats, it can be concluded that the plant extract has anti-diabetic effect, resulting in antidiabetic effects is the dose-dependent manner (Fig. 3). Moreover, it can be concluded from the results that a dose of 20 mg/kg BW in the first four days of treatment had a blood-glucose-lowering effect compared to the control group. However, because of drug resistance in the animals, the decrease in blood glucose in the following treatment days was not effective even caused increased blood glucose levels, and also, the effect of reducing blood glucose levels in 40 mg/kg group is the same as the positive control group.
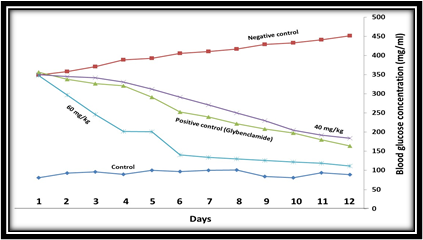
Figure 3. The effect of single oral dose 40 and 60 mg/kg BW of hydroalcoholic extract of P. farcta in STZ-induced diabetic rats. Plasma glucose level was significantly decreased in 40 and 60 mg/kg BW dose after the 5 days dose administration as well as 8 ml/kg Subcutaneous (p<0.001).
Effect of intraperitoneal injection of the aqueous extract of P. farcta on reducing blood glucose
In order to investigate the effect of the medicinal plant on reducing blood glucose and eliminating the ambiguities associated with the absorption of intestines, 8 ml/kg body weight of the herb extract was administrated intraperitoneally on streptozotocin-induced diabetic rats in 12 days. Administration of 2 ml of 60 mg/kg BW dosage concentrated extract on the third day of treatment caused a significant decrease in blood glucose levels in animals compared to the control group (Figure 4) (P <0.001). The findings of this study indicate that the extract of P. farcta is effective in decreasing blood glucose in diabetic animal models both in oral administration and in peritoneal injection. Moreover, the decrease in blood glucose associated with the medicinal plant is dose-dependent.
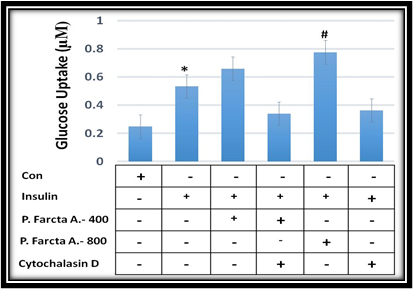
Figure 4. Effect of intraperitoneal injection of the extract of P. farcta on reducing blood glucose. Plasma glucose levels were significantly decreased in 8 ml/kg BW dose in subcutaneous after the 5-days dose administration as well as 40 mg/kg orally but the decreasing effect is not as much as 60 mg/kg orally. (p<0.001).
P. farcta aqueous extract induced glucose uptake in 3T3 L1 adipocyte cells
Differentiation of 3T3-L1 adipocytes plays an important role in biological functions. About 5 days after FBS reduction, the differentiated cells were tested for 2-deoxyglucose uptake. In the absence of insulin stimulation, the glucose uptake through the cell membrane was minimal in 3T3 L1 adipocyte cells. There was an increase in glucose uptake on the cell surface after 20 min incubation with insulin stimulation (Figure 5). We then studied the effects of aqueous extract of P. farcta on glucose uptake by 3T3 L1 adipocyte cells. There was an increase in glucose uptake on the cell surface after 30 min incubation with 400 and 800 µg/ml of P. farcta extract. Quantitative colorimetric measurements showed that P. farcta extract significantly increased insulin-stimulated glucose uptake in 3T3 L1 adipocyte cells (Figure 5).
Insulin-triggered glucose uptake inhibited by actin filament cytoskeleton inhibitor
As actin remodeling is closely associated with glucose uptake, which is markedly stimulated by insulin, we studied the effects of cytochalasin D when interfered with actin cytoskeleton function. We found that insulin-stimulated glucose uptake was markedly decreased in cells treated with actin filament polymerization inhibitor (Figure 5). Insulin-dependent stimulation of glucose uptake was completely blocked by treatment with 1 µM cytochalasin D for 30 min. Quantitative colorimetric measurements showed that cytochalasin D significantly blocked insulin-stimulated glucose uptake in 3T3 L1 adipocyte cells (Figure 5).
Figure 5: The effect of P. farcta fruit extract at the doses of 400 and 800 µg/ml along with cytochalasin- D (actin filaments polymerization inhibitor) on glucose uptake in 3T3 L1 adipocyte cells. * P≤0.001, # P≤0.001.
DISCUSSION
One of the major problems that new medicine brings with its obvious advantages over traditional medicine, is the increasing consumption of chemical drugs, which, unfortunately, takes on more acute forms day after day. In terms of the implications of this issue, two very important issues are worth mentioning. First of all, due to continuous, over-the-counter and sometimes no-particular consumption of some chemical drugs, the phenomenon of self-defense and drug resistance in germs and viruses gradually develops, thereby the drug effect is reduced and thus the patient increases the drug doses and turns into more potent types of it. Secondly, although the use of chemical drugs in certain diseases is desired, long-term use and, in some cases, even certain effects are sometimes referred to as side effects and can be also more dangerous than the disease itself [14]. Drug resistance is the reduction of a drug’s effect in the treatment of a disease which increases the sustainability of a drug marker and drug resistance to various types of pathogens including bacteria, parasites, viruses, fungi, and cancer cells. When the pathogen is resistant to more than one compound, this phenomenon is called "multi-drug resistance" [15]. Insulin resistance is a condition in which body cells are resistant to the insulin hormone. This disease may be a part of the metabolic syndrome which is associated with a high risk of heart disease. Insulin resistance increases the risk of developing type 2 diabetes. Although there are genetic risk factors for the disease, insulin resistance can be controlled by a proper diet, exercise, and proper medications.
The insulin resistance may be against the insulin, produced in the body (endogenous) or to the injectable insulin (exogenous). Insulin resistance makes the pancreas produce more and more insulin until it is able to produce enough insulin for the body. At this time, blood glucose goes up and turns to an important risk factor for diabetes and heart disease. If a person has three or more of the following features, he has this syndrome: Fat accumulation in the chamber (apple obesity), in which over-the-counter amount is more than 40 inches (102 centimeters) for men and more than 35 inches (88 centimeters) for women. High levels of triglyceride (> 150 mg/dL); HDL cholesterol: less than 40 mg/dl in men and less than 50 mg/dl in women; Blood pressure 130/85 mmHg or more; and high levels of fasting blood glucose indicator for insulin resistance of 110 mg/dl or more [14]. Diabetes Mellitus is a chronic, lifelong disease, and one of the most common endocrine disrupters, which is due to lower levels of insulin secretion by beta cells in the Langerhans. Type I diabetes means the body is still able to produce some insulin, but this is not enough. Type II diabetes occurs when produced insulin does not function properly for two reasons: First, insulin is unable to send its message through a membrane receptor to the cell, which is the negative regulation of the insulin receptor. Secondly, if the receptor receives a correct function and the insulin message arrives the cell, the probability of deficiency is the reduction of a message transmission pathway protein, which transfers glucose from the cell onto the membrane, leading to increasing the blood glucose and reducing the absorption of glucose from the blood by the cell. Type II diabetes is treated with a healthy diet and increased physical activity. In addition, it often requires medication or insulin. It's very important for diabetics to control their blood sugar, blood pressure, and lipids properly to reduce the risk of side effects. It is characterized by metabolic disorders and long-term complications in the eyes, kidneys, nerves and blood vessels, as well as it is associated with a large disruption of carbohydrate, lipids, proteins, water, and electrolytes metabolisms [14, 15]. Considering the many and sometimes fatal cases of illness in diabetics, it is felt necessary to consider ways to treat, discourage and prevent it. Treatment modalities, currently available for the treatment of diabetes, such as dietary changes and oral hypoglycemic agents, have limitations [15]. Herbal medicine is used throughout the world for a range of people with diabetes and the study of medicinal herbs provides a natural key to unlock the health problems of the disease.
The results of this study indicate that the injection of streptozotocin 50 mg/kg intraperitoneally induces a type II diabetic model in rats so the glucose level in diabetic rats was significantly higher than that in the control group. In this study, the effect of hydroalcoholic extract of P. farcta fruit on the level of glucose in male Wistar rats was investigated and a comparison was also made between the effect of the extract and the chemical agent, Glibenclamide in diabetes. The results of the present study indicate that oral administration of the extract significantly reduces the glucose level. The effect of plant extract on the blood glucose level is in a dose-dependent manner. So that the dose of 10 mg/kg body weight did not significantly reduce blood glucose levels in experimental groups, but a dose of 20 mg/kg of body weight could reduce blood glucose levels till the fourth day and after 4th days it was not able to reduce to glucose levels, which may be due to the drug resistance.
like 20 mg dose, a dose of 40 mg/kg of body weight, was able to reduce blood glucose levels by the fourth day and had a greater effect on blood glucose levels since the fourth day rather than the 20 mg dose. In fact, the difference between the doses of 20 and 40 mg from the fourth day was significant and against the dose of 20 mg. The effects of 60 mg/kg body weight, 72 hours after administration of the extract, showed a significant decrease in hypoglycemic effect than glibenclamide. It was also determined that administration of 2 ml of this extract by intraperitoneal injection in the streptozotocin-induced diabetic rats significantly reduced blood glucose levels. The effect of hypoglycemic intraperitoneal injection of the extract was somewhat similar to and more potent than glibenclamide, but its hypoglycemic effects were not more effective than orally received 60 mg/kg.
On the other hand, 3T3-L1 adipocyte cells were exposed at the aqueous extract of P. farcta fruit at the doses of 400 and 800 µg/ml, and glucose uptake was investigated. The results showed that in the presence of any stimulator “as a control group” the cells had no reaction, but in the presence of 10 nM insulin, there was a significant increase in the glucose uptake. Data analysis using Chi-square test showed that by increasing the concentration of aqueous extract of P. farcta, the percentage of glucose uptake in 3T3-L1 adipocyte cell line had increased with a significant correlation (p>0.001); which 800 µg/ml of the aqueous extract of P. farcta had the highest glucose uptake level in 3T3-L1 adipocyte cells by a concentration-dependent manner which was a significantly lower glucose uptake with cytochalasin D (P<0.001). Regarding the results of the antidiabetic drug effects of the extract with two oral and intraperitoneal injections, in which oral route showed more effective result, as well as cell culture method, it can be concluded that the anti-diabetic effects of this plant through the effect on the digestive tract system has a function similar to that of metformin. It should be noted that the medicinal effect of the fruit extract on diabetes has not been studied much and it can be said that it was the first time that an in vivo study was conducted on diabetic rats and there is a need to the results of supplementary studies to reach a logical reason to introduce P. farcta as an anti-diabetes medicine.
REFERENCES

