



|
Antibacterial Activity and Major Constituents of Some Essential Oils from Aromatic Plants Manal Jameel Kiki 1*, Ghada Saber Ibrahim 2, 3 |
|
1 The University of Jeddah, College of Science, Department of Biology, Jeddah, Saudi Arabia. 2 The University of Jeddah, College of Science, Department of Biochemistry, Jeddah, Saudi Arabia. 3 Microbial Biotechnology Department, Genetic Engineering and Biotechnology Research Division, National Research Centre, 33 Bohouth St., Dokki, Giza, 12622, Egypt.
|
ABSTRACT
The use of natural products such as antibacterial compounds has increased an effort to reduce the prevalence of bacteria, mostly antibiotic-resistant pathogens. The purpose of this study was to assess the variability in chemical components and antibacterial effectiveness of four essential oils (EOs) from aromatic plants: Thyme (Th), Marjoram (Ma), Mint (Mi), and Dill (Di) against some pathogenic bacteria. Gas chromatography-mass spectrometry (GC-MS) techniques were used to determine the majority of oil contents. The disk diffusion technique was performed to test antibacterial activity; subsequently, the modified agar-well diffusion method was used to assess minimum inhibitory concentration for oils. The antibacterial activity performed against the standard strains Staphylococcus aureus (ATCC 29213), Staphylococcus epidermides (ATCC 12228), Enterococcus faecalies (ATCC 29212), Pseudomonas aeruginosa (ATCC 27853), Klebsiella pneumonia (ATCC 700603), and Escherichia coli. All tested essential oils showed effective antibacterial activity, particularly Th-EO which exhibited a strong antibacterial effect where the results showed that Th-EO revealed high activity against all pathogenic strains, and demonstrated a good efficacy against the antibiotics resistant strain P. aeruginosa. The results showed also that Th-EO possesses the greater antibacterial activity than other EOs with larger inhibition zones (40 mm) and lower minimum inhibitory concentrations less than (2.0 mg/ml). The main components of the Th-EO were Thymol, Benzene, 1-methyl-3-(1-methylethyl), and gamma.-Terpinene. According to the results, essential oils may represent a natural forceful source of compounds with applications in the pharmaceutical and food industries.
Key Words: Essential oils, Aromatic plants, Chemical composition, Antibacterial activity, MIC.
INTRODUCTION
Searching for additional sources of active antimicrobial agents and methods for treating severe bacterial infections is critical nowadays due to the widespread of multidrug-resistant pathogens. The carcinogenicity and toxicity of synthetic additives have led food scientists to look for alternatives that occur especially naturally as antimicrobials [1]. Instead of synthesizing products, there is a growing interest in using antimicrobials and other medicines extracted from plants. Initial screening of possible plant-based antibacterial and antifungal compounds may achieve using pure substances or crude extracts [2]. The relieving effects of many traditional herbs and spices indicate the presence of antioxidants and antimicrobials within [3]. EOs are complex mixtures that consist of several individual compounds and each of these constituents causes beneficial or harmful effects of these oils. The biological effect of EOs depends on their chemical compounds, which is specified by the plant genotype and strongly affected by a variety of factors, such as environmental and agriculture conditions of geographical origin [4,5].
Thyme (Thymus vulgaris) is widespread and has a valuable remedy that has been used for centuries and has been recognized as a useful resource of vitally active compounds with substantial antioxidant and anti-inflammatory properties. These compounds are potentially valuable for the prevention and treatment of pathogenic [6]. The major components of the EO found to be Thymol and carvacrol, and have an antimicrobial effect against fungi, gram (+) and (-) bacteria [7, 8]. Thyme also has numerous beneficial properties, such as antiseptic, carminative, antimicrobial, and antioxidant [9]. The Thymus derived EOs have already demonstrated antimicrobial activity, which correlates with the thymol content of the oil [10].
Dill (Antheum graveolens) is a widespread herbal aromatic found in the Umbelliferae family. EO and dill extracts have documented varying degrees of antimicrobial activity, which may be due to the presence of furanocoumarin in dill [11]. Limonene and carvone, have exhibited intense antifungal activity against A. niger, S. cerevisiae, and C. albicans [11, 12]. In general, Gram (-) bacteria are more resistant to the impacts of essential oils than a gram (+). It limits the diffusion of hydrophobic compounds through its covering lipopolysaccharides, but this was not always true [13, 14]. Peppermint (Mentha piperita) is a very beneficial and essential plant. It is used in food, cosmetics, and medicines [15]. Peppermint oil demonstrated antibacterial activities against Xanthamonas campestris, P. aeruginosa, Salmonella typhimurium, and E. coli [16].
Marjoram (Origanum majorana) is one plant of the family Lamiaceae, and among many essential oils that may be useful as antimicrobials, marjoram oil has antimicrobial properties against foodborne bacteria and mycotoxigenic fungi. It may, therefore, have the most significant potential for industrial usage [17].
This research aims to determine the antimicrobial activity and minimal inhibitory concentration (MIC) of the essential oils from four aromatic plants investigated, also, to investigate the composition of these oils through Gas-chromatography/mass spectrophotometrical analyses.
MATERIALS AND METHODS
Essential Oil: Thyme, Marjoram, Mint, and Dill EOs were purchased from a local market in Jeddah, Saudi Arabia.
Tested bacterial strains: Gram (+) bacteria: S. aureus (ATCC 29213), S. epidermides (ATCC 12228), E. faecalies (ATCC 29212), and Gram (-) bacteria: P. aeruginosa (ATCC 27853), K. pneumonia (ATCC 700603), E. coli.
Essential oils analysis by GC-MS
GC-MS (Agilent Technologies) device fitted with a GC (7890B) and Ms Detector (5977A). The samples were hexane-diluted (1:19, v / v). The GC is equipped with an HP-5MS column (internal diameter 30 m x 0, 25 mm and film thickness 0, 25 μm). Analyze conducted by the same condition that used by Ibrahim and Kiki, 2020 [18]. Identification of various constituents calculated by comparing spectrum fragmentation patterns with data stored in the Wiley and NIST mass spectral libraries.
Antibacterial activity
The standard diffusion method by discs was used [19], and the bacterial strain was cultivated for 18h at 37 °C in each experiment. 100μL of the bacterial suspension was spread out on Mueller Hinton Agar. Sterile disks (6 mm) were placed on a Petri plate and saturated with 10 μL of EO, then left for 30 min. The discs are placed on the surface of the inoculated Petri plate. Subsequently, incubated plate at 37 °C for 24 h and the inhibition zones were determined in mm. Three replicates of the assays were performed, and the average was taken.
Determination of minimal inhibitory concentration
(MIC) value estimated by the modified agar diffusion procedure [20]. DMSO has prepared two-fold serial dilutions for EOs of thyme, marjoram, mint, and dill to achieve a decreasing concentration range from 128 mg/ml to 2 mg/ml in the agar-well diffusion technique. Bacteria spread over the plate of the Mueller Hinton Agar, then 100 μl of critical oil dilutions filled the wells. The bacterial cultures incubated at 37 ° C for 24 h. The lowest concentration for all EOs viewing a clear inhibition zone has been taken as MIC. DMSO and Amoxicillin (100µg/ml) were used as a negative and positive control, respectively. Three replicates of the assays performed and the average was taken.
RESULTS AND DISCUSSION
Chemical compositions of essential oils
Table (1) showed the chemical constituents and the relative percentage of the total chromatogram area of Th-EO, Ma-EO, Mi-EO, and Di-EO. The first one was Th-EO, where eight compounds were identified, representing 99.99 % of the total Th-EO. The major compounds were Thymol (45.16%), Benzene, 1-methyl-3-(1-methylethyl) - (29.22%), gamma.-Terpinene (23.65%) and Benzene, 1-methyl-4-(1-methylethyl) (1.03%). Minor components were α- pinene (0.34%), camphene (0.28%), Cyclohexene, 1-methyl-4-(1-methylethyl) (0.11%), and Phenol, 5-methyl-2-(1-methylethyl) (0.2%).These results are in agreement with Porte and Godoy, (2008) [21], who reported that the major compounds in the Th-EO were Thymol (44.7 %), p-cymene (18.6 %), and γ-terpinene (16.5 %).
The next EO was Marjoram, and the results illustrated that Ma-EO contained five compounds that represent 100 % of the total Ma-EO. The major compounds were Eucalyptol (73.57 %), alpha.-Terpineol (9.25 %), beta.-Pinene (6.34%). Alpha-pinene (5.79%) and Linalool (5.05%). The variations in compounds of marjoram EO could be due to many reasons, such as species, stage of growth, the herb origin, climatic, and drying methods [22, 23].
That Mint EO (Mi-EO) which contained thirteen compounds represent 100 % of total Mi-EO. The major compounds were Cyclohexanone, 5-methyl-2-(1-methylethyl) - (53.67 %), l-Menthone (24.66 %), Cyclohexanol, 5- methyl-2-(1-methylethyl)-, (1.alpha.,2.beta.,5.beta.) (9.32%), Isopulegol (2.94%), Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1.alpha, 2.beta, 5.beta.)- (2.46%), Caryophyllene (1.81%) and Levomenthol (1.55%).
Previous studies demonstrated that menthol is the most critical constituents of Mi-EO [16, 24], and the menthone and limonene were the second of Mi-EO [16, 24]. Still, one study showed that piperitone oxide, α- terpinene, was the main component of Mi-EO [30]; for that reason menthol is also not always the main constituent of Mi-EO. From this study, the chemical part of Mi-EO is comparable with Iscan et al., (2002) [16].
Dill was the last EO, where eleven compounds found, representing 99.99% of the total Di-EO. Alpha.-Phellandrene (32.67%), D-Limonene (22.67%), 3, 6-Dimethyl-2, 3, 3a, 4, 5,7a-hexahydrobenzofuran (12.78%), Apiol (9.99%) and Carvone (9.51%) were the main compounds. The chemical composition of the volatile Di-EO differed according to the parts of the plant. Based on their chemical composition, the analysis of EOs from dried flowers, leaves, and dill fruits grown in Romania, where found that percentage of α-phellandrene and limonene in leaves (62.71 and 13.28%) and flowers (32.26 and 33.22%). The main ingredients in fruit essential oil were carvone (1.75.21 %), with 21.56 and 0.12 % of phellandrene and limonene [26]. Santos et al., (2002) [27] analyzed differences in the chemical composition of EOs from the parent plant's fruit, aerial parts, roots, and hairy dill root crop and were found that Carvone (67%) and limonene (23%) were the major components of the dill fruit oil, while α-phellandrene dominated the herb oil (62%).
Antibacterial activity and minimum inhibitory concentration
Antibacterial activity of Thyme, Marjoram, Mint, and Dill EOs against pathogens Gram (+) bacteria S. aureus (ATCC 29213), S. epidermides (ATCC 12228), E. faecalies (ATCC 29212), and Gram (-) bacteria P. aeruginosa (ATCC 27853), K. pneumonia (ATCC 700603) and E. coli by using disc diffusion technique were reported in (Table 2). The results demonstrated that (Th-EO) revealed an antibacterial effect against all tested pathogens bacteria (Fig.1, 2), while other EOs showed variation as antibacterial agents with tested pathogens. The maximum antibacterial activity of Th-EO is shown in the diameter of the inhibition zone (40 mm) with S. aureus (ATCC 29213), S. epidermides (ATCC 12228), and E. coli.
The maximum antibacterial activity of Ma-EO shown in the diameter of inhibition zone (16 mm) with E.coli followed by Staphylococcus aureus (ATCC 29213) (13mm) (Fig.1), S. epidermides (ATCC 12228) (12mm) (Fig.2 A) and K. pneumonia (ATCC 700603) (8mm), while P. aeruginosa (ATCC 27853) and E. faecalies (ATCC 29212) showed complete resistance to Ma-EO as an antibacterial agent (Fig.2 B).
Mi-EO showed maximum inhibition zone with E.coli (20mm) followed by S. aureus (ATCC 29213) (18mm) (Fig. 1), S. epidermides (ATCC 12228) (12mm), and E. faecalies (ATCC 29212) (8mm), Where P. aeruginosa (ATCC 27853) and K. Pneumonia (ATCC 700603) showed complete resistance to Mi-EO as an antibacterial agent. Di-EO showed maximum inhibition zone with S. aureus (ATCC 29213) and E. coli (14mm), where E. faecalies (ATCC 29212), P. aeruginosa (ATCC 27853), and K. pneumonia (ATCC 700603) showed complete resistance to Di-EO as an antibacterial agent.
The inhibition zone generated by (Thyme, Marjoram, Mint, and Dill) EOs were confirmed by the inhibitory potential of these EOs through estimating the (MIC) which is determined by preparing serial dilution from the essential oil (20 to 128 mg/mL). The results of the EOs MIC were summarized in (Tables 3, 4, 5, and 6). Th-EO showed a significant inhibitory effect against tested bacteria (Table 3) where all microbial strains inhibited at 16 mg/ml. P. aeruginosa is resistant to this oil with a MIC less than (2mg/ml) in compression to the positive control Amoxicillin, where it showed complete resistance when it was used at (100µg/ml). Whereas S. aureus, K. pneumonia, and E. coli have the lowest MIC at (4 mg/ml), while S. epidermides (8mg/ml) and E. faecalies (16mg/ml).
Ma-EO showed a significant inhibitory effect against some studied bacterial, where E. faecalies and P. aeruginosa showed a complete resistance. K. pneumonia is more resistant to this oil with a MIC of more than (128mg/ml), whereas S. epidermides and E. coli have the lowest MIC (16 mg/ml) with this oil, while S. aureus MIC was (128mg/ml). The mint essential oil showed MIC value of (16 mg/ml) against S. aureus, S. epidermides, E. faecalies, and E. coli. In contrast, P. aeruginosa and K. pneumonia showed complete resistance to this oil. Di-EO showed the lowest antibacterial effect on the studied strains. The results in (Table 6) illustrated that the lowest MIC of Di-EO was (4mg/ml) against S. epidermides, while it was (16mg/ml) with S. aureus and E. coli. At the same time, E. faecalies, P. aeruginosa, and K. pneumonia showed complete resistance to Di-EO.
Several other studies have reported comparable findings within the literature with those we obtained about Th-EO antibacterial activity [28, 29]. Ivanovic et al., (2013) [30] reported significant activity of thyme extract and EO against E. coli and Salmonella varieties, at MIC (640 μg/ml). The high concentration of Thymol in the extract (39.7 %) and essential oil (48.49 %) was due to such activity. It also reported the antimicrobial activity of Th-EO 5% (V/V) against E. coli and other foodborne bacteria [31]. This study is in agreement with our review, where Th-EO contains 45.15% thymol.
Various mechanisms correlated with EOs antimicrobial activity were suggested such as bacterial cell-wall degradation, modification of membrane proteins, changes in membrane permeability, enzymes inactivation, decreasing ATP, and cellular leakage [32-34]. Some research, however, documented the particular mechanism of action for individual constituents of the oil. In several studies, EOs contain Phenolic-structural elements such as carvacrol, Thymol, and eugenol have strong antimicrobial efficiency [35, 36].
Several mechanisms for describing their mechanism of action are proposed. The eugenol hydroxyl group may react with proteins and inhibit enzyme action; hydrophobic carvacrol and thymol can destroy the gram (-) bacterial cell walls and discharge lipopolysaccharides [37]. However, it commonly believed that an essential oil's antimicrobial effectiveness is not entirely correlated with a single compound, but with a synergic influence of all components.
CONCLUSION
The essential oils from the aromatic plants (Thyme, Marjoram, Mint, and Dill) proved to be a promising source of biomolecules with potential antibacterial activity, where Th-EO possesses energetic antibacterial activities which can represent as natural antibacterial with efficient applications in the food and pharmaceutical industries.
Conflicts of interest
The authors declare that they have no conflicts of interest.
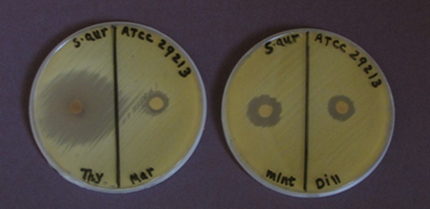
Fig. 1. Antibacterial activities of (Thyme, Marjoram, Mint, and Dill) EOs against Staphylococcus aureus by using disc diffusion method after 24 h of incubation at 37 °C.
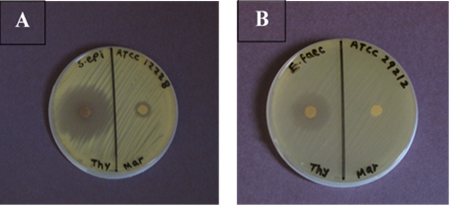
Fig.2. Antibacterial activities of (Thyme, Marjoram) EOs agains (A) Staphylococcus epidermidis (B) Enterococcus faecalis by using disc diffusion method after incubation at 37 °C for 24 h.
Table 1. Chemical composition of Thyme, Marjoram, Mint, and Dill EOs.
|
Identified compounds |
Relative area % |
|||
|
Thyme |
Marjoram |
Mint |
Dill |
|
|
Alpha.-Pinene, (-)- |
0.34 |
5.79 |
- |
2.01 |
|
Camphene |
0.28 |
- |
- |
- |
|
Cyclohexene, 1-methyl-4-(1-methylethyl)-, (R)- |
0.11 |
- |
- |
- |
|
Benzene, 1-methyl-3-(1-methylethyl)- |
29.22 |
- |
- |
- |
|
Benzene, 1-methyl-4-(1-methylethyl)- |
1.03 |
- |
- |
- |
|
gamma.-Terpinene |
23.65 |
- |
- |
- |
|
Phenol, 5-methyl-2-(1-methylethyl)- |
0.2 |
- |
- |
- |
|
Thymol |
45.16 |
- |
- |
- |
|
(-)-.beta.-Pinene |
- |
6.34 |
- |
- |
|
Eucalyptol |
- |
73.5 |
- |
- |
|
Linalool |
- |
5.05 |
- |
- |
|
alpha.-Terpineol |
- |
9.25 |
- |
- |
|
dl-Limonene |
- |
- |
0.31 |
- |
|
Isopulegol |
- |
- |
2.94 |
- |
|
l-Menthone |
- |
- |
24.66 |
- |
|
Cyclohexanone, 5-methyl-2-(1-methylethyl)- |
- |
- |
53.67 |
- |
|
Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1.alpha.,2.beta.,5.beta.)- |
- |
- |
9.32 |
- |
|
Levomenthol |
- |
- |
1.55 |
- |
|
Cyclohexanone, 5-methyl-2-(1-methylethylidene)- |
- |
- |
0.75 |
- |
|
2-Cyclohexen-1-one, 3-methyl-6-(1-methylethyl)- |
- |
- |
1.51 |
1.57 |
|
Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1.alpha.,2.beta.,5.beta.)- |
- |
- |
2.46 |
- |
|
(-)-.beta.-Bourbonene |
- |
- |
0.54 |
- |
|
BETA.-ELEMENE |
- |
- |
0.21 |
- |
|
Caryophyllene |
- |
- |
1.81 |
- |
|
Alloaromadendrene |
- |
- |
0.27 |
- |
|
alpha.-Phellandrene |
- |
- |
- |
32.6 |
|
Benzene, 1-methyl-4-(1-methylethyl)- |
- |
- |
- |
6.59 |
|
D-Limonene |
- |
- |
- |
22.67 |
|
(+)-2-Bornanone |
- |
- |
- |
0.38 |
|
3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran |
- |
- |
- |
12.78 |
|
Carvone |
- |
- |
- |
9.52 |
|
2-Oxabicyclo[2.2.2]octan-6-ol, 1,3,3-trimethyl acetate |
- |
- |
- |
1.55 |
|
Apiol |
- |
- |
- |
9.99 |
|
3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran |
- |
- |
- |
12.78 |
Table 2. Antibacterial activity of (Thyme, Marjoram, Mint, and Dill) EOs against tested bacterial strains using disc diffusion method.
|
Bacterial strain |
Inhibition zone ( mm ) formed by essential oil |
|||
|
Thyme |
Marjoram |
Mint |
Dill |
|
|
S. aureus |
40 |
13 |
18 |
14 |
|
S. epidermides |
40 |
12 |
12 |
7 |
|
E. faecalies |
24 |
R |
8 |
R |
|
P. aeruginosa |
10 |
R |
R |
R |
|
K. pneumonia |
12 |
8 |
R |
R |
|
E. coli |
40 |
16 |
20 |
14 |
.
(R) resistant
Table 3. MIC of Thyme EO against tested bacterial strains.
|
Bacterial strain |
Concentration of Th-EO (mg/ml) |
||||||||
|
2 |
4 |
8 |
16 |
32 |
64 |
128 |
PC |
NG |
|
|
S. aureus
|
+ |
-
|
- |
- |
- |
- |
- |
- |
+ |
|
S. epidermides
|
+ |
+ |
- |
- |
- |
- |
- |
- |
+ |
|
E. faecalies
|
+ |
+ |
+ |
- |
- |
- |
- |
- |
+ |
|
P. aeruginosa
|
- |
- |
- |
- |
- |
- |
- |
+R |
+ |
|
K. pneumonia
|
+ |
- |
- |
- |
- |
- |
- |
- |
+ |
|
E. coli
|
+ |
- |
- |
- |
- |
- |
- |
- |
+ |
(PC) positive control (Amoxicillin), (NC) negative control (DMSO), (R) resistant, (+) grew, (-) not grew.
Table 4. MIC of Marjoram EO against tested bacterial strains.
|
Bacterial strain |
Concentration of Ma-EO (mg/ml) |
|||||||||
|
2 |
4 |
8 |
16 |
32 |
64 |
128 |
PC |
NG |
|
|
|
S. aureus
|
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
|
|
S. epidermides
|
+ |
+ |
+ |
- |
- |
- |
- |
- |
+ |
|
|
E. faecalies
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
+ |
|
|
P. aeruginosa
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+R |
+ |
|
|
K. pneumonia
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
+ |
|
|
E. coli
|
+ |
+ |
+ |
- |
- |
- |
- |
- |
+ |
|
PC) positive control (Amoxicillin), (NC) negative control (DMSO), (R) resistant, (+) grew, (-) not grew.
Table 5. MIC of Mint EO against tested bacterial strains.
|
Bacterial strain |
Concentration of Mi-EO (mg/ml) |
||||||||
|
2 |
4 |
8 |
16 |
32 |
64 |
128 |
PC |
NG |
|
|
S. aureus
|
+ |
+ |
+ |
- |
- |
- |
- |
- |
+ |
|
S. epidermides
|
+ |
+ |
+ |
- |
- |
- |
- |
- |
+ |
|
E. faecalies
|
+ |
+ |
+ |
- |
- |
- |
- |
- |
+ |
|
P. aeruginosa
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+R |
+ |
|
K. pneumonia
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
+ |
|
E. coli
|
+ |
+ |
+ |
- |
- |
- |
- |
- |
+ |
(PC) positive control (Amoxicillin), (NC) negative control (DMSO), (R) resistant, (+) grew, (-) not grew.
Table 6. MIC of Dill EO against tested bacterial strains.
|
Bacterial strain |
Concentration of Di-EO (mg/ml) |
||||||||
|
2 |
4 |
8 |
16 |
32 |
64 |
128 |
PC |
NG |
|
|
S. aureus
|
+ |
+ |
+ |
- |
- |
- |
- |
- |
+ |
|
S. epidermides
|
+ |
- |
- |
- |
- |
- |
- |
- |
+ |
|
E. faecalies
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
+ |
|
P. aeruginosa
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+R |
+ |
|
K. pneumonia
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
+ |
|
E. coli
|
+ |
+ |
+ |
- |
- |
- |
- |
- |
+ |
(PC) positive control (Amoxicillin), (NC) negative control (DMSO), (R) resistant, (+) grew, (-) not grew.
REFERENCES
[1] Feng W, Zheng X. Essential oils to control Alternaria alternate in vitro and in vivo, Food Control, 2007; 18: 1126-1130.
https://doi.org/10.1016/j.foodcont.2006.05.017
[2] Carovic-Stanko K, Politeo S, Strikic O, Kolak F, Milos I. Composition and antibacterial activities of essential oils of seven Ocimum taxa, Food Chemistry, 2010; 119: 196-201.
[3] Ghaima KK, Hashim NM, Ali SA. Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale), J. Appl. Pharm. Sci., 2013; 3: 96-99.
https://doi.org/10.7324/JAPS.2013.3518.
[4] Rota C, Carraminana J, Burillo J, Herrera A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens, Food Protec., 2004; 67: 1252–1256.
https://doi.org/10.4315/0362-028X-67.6.1252.
[5] Yesil CO, Hames KE, Bedir E, Vardar SF, Ozek T, Baser K. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations, Food Chem., 2007; 100: 553–559. DOI: 10.1016/j.foodchem.2005.10.011.
[6] Dehimi K, Speciale A, Saija A, Dahamna S, Raciti R, Cimino, Cristani M. Antioxidant and anti-in-flammatory properties of Algerian Thymelaea microphylla Coss. And Dur. Extracts, Pharm. Mag., 2016; 12 (47): 203-210.
DOI: 10.4103/0973-1296.186345.
[7] Perruci S, Mancianti F, Cioni PL, Flamini G , Morelli I, Macchioni G. In Vitro Antifungal Activity of Essential Oils Against Some Isolates of Microsporum Canis and Microsporum Gypseum, Planta Med., 1994; 60: 184. DOI: 10.1055/s-2006-959448.
[8] Ismaiel A, Pierson MD. Inhibition of Growth and Germination of C. botulinum 33A, 40B, and 1623E by Essential Oil of Spices, J. Food Sci., 1990; 55: 1676. https://doi.org/10.1111/j.1365 2621.1990.tb03598.x
[9] Baranauskiene R, Venskutonis PR, Viskelis P, Dambrauskiene E. Influence of nitrogen fertilizers on the yield and composition of thyme (Thymus vulgaris), Journal of Agricultural and food chemistry, 2003;51: 7751-58. https://doi.org/10.1021/jf0303316.
[10] Didry N, Dubreuil L, Pinkas M. Antibacterial activity of thymol, carvacrol and cinnamaldehyde alone or in combination. Pharmazie, 1993; 48: 301-304.
[11] Stravri, M, Gibbons S. The antimycobacterial constituents of dill (Anethum graveolens), Phytoterapy Research, 2005; 19: 938-941.
https://doi.org/10.1002/ptr.1758.
[12] Kaur GJ, Arora DS. Bioactive potential of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi belonging to the family Umbelliferae-current status, Journal of Medicinal Plants Research, 2010; 4 (2): 087094.
http://www.academicjournals.org/JMPR.
[13] Angienda PO, Onyango DM, Hill DJ. Potential application of plant essential oils at sublethal concentrations under extrinsic conditions that enhance their antimicrobial effectiveness against pathogenic bacteria, African Journal of Microbiology Research, 2010; 4 (16): 1678-1684.
[14] Sonali J, Shekhawat GS. Phytochemical analysis and antibacterial screening of in vivo and in vitro extracts of Indian medicinal herb: Anethum graveolens, Research Journal of Medicinal Plant, 2010; 4 (4): 206-212.
DOI: 10.3923/rjmp.2010.206.212.
[15] Scavroni J, Boaro CSF, Marques MOM, Ferreira LC. Yield and composition of the essential oil of Mentha piperita L. (Lamiaceae) grown with biosolid, Brazilian Journal of Plant Physiology, 2005; 17(4): 1677-1679.
https://doi.org/10.1590/S167704202005000400002.
[16] Iscan G, Kirimer N, Kurkcuoglu M, Baser KH, Demirci F. Antimicrobial Screening of Mentha piperita Essential Oils, Journal of Agricultural and Food Chemistry, 2002; 50: 3943-3946.
https://doi.org/10.1021/jf011476k.
[17] Mohamed MH, Mansour H.A, Incorporating essential oils of marjoram and rosemary in the formulation of beef patties manufactured with mechanically deboned poultry meat to improve the lipid stability and sensory attributes, LWA-Food Science and Technology, 2012;45: 79-87.
https://doi.org/10.1016/j.lwt.2011.07.031.
[18] Ibrahim GS, Kiki MJ. Chemical Composition, Antifungal and Antioxidant Activity of Some Spice Essential Oils, International Journal of Lifescience and Pharma Research, 2020; 10 (1): 45-52.
http://dx.doi.org/10.22376/ijpbs/lpr.2020.10.1.L45-52
[19] Bauer AW, Kirby WMM, Sherries JC. Tuck M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method, Am J Clin Path., 1966; 45:493-496.
[20] Okeke MI, Iroegbu CU, Eze EN, Okoli AS, Esimone CO. Evaluation of extracts of the root of Landolphia owerrience for antibacterial activity, Journal of Ethnopharmacology, 2001; 78: 119-127.
https://doi.org/10.1016/S0378-8741. (01)00307-5.
[21] Porte A, Godoy RLO. Chemical composition of Thymus vulgaris L. (thyme) essential oil from the Rio de Janeiro State (Brazil), J. Serb. Chem. Soc., 2008; 73 (3): 307–310.doi: 10.2298/JSC0803307P.
[22] Sellami IH, Maamouri E, Chahed T, Wannes WA, Kchouk ME, Marzouk B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.), Industrial Crops and Products, 2009; 30: 395-402.
https://doi.org/10.1016/j.indcrop.2009.07.010.
[23] Baatour O, Tarchoune I, Mahmoud H, Nassr NW, Kaddour R, Hamdaou G, Ayachi MBN, Nasri BM, Lachaal M, Marzouk B. Culture conditions and salt effects on essential oil composition of sweet marjoram (Origanum majorana) from Tunisia, Acta Pharm., 2012; 62: 251-261. doi: 10.2478/v10007-012-0019-9.
[24] Hussain AI, Anwar F, Nigam PS, Ashraf M, Gilani AH. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species, Journal of the Science of Food and Agriculture, 2010; 90: 1827-1836. https://doi.org/10.1002/jsfa.4021.
[25] Yadegarinia D, Gachkar L, Rezaei MB, Taghizadeh M, Alipoor Astaneh SH, Rasooli I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry, 2006. 67, 1249-1255.
https://doi.org/10.1016/j.phytochem.2006.04.025
[26] Radelescu V, Popescu ML, Ilies D. Chemical composition of the volatile oil from different plant parts of Anethum graveolems L. (Umbelliferae) cultivated in Romania, Farmacia, 2010; 58 (5):594-600.
https://www.researchgate.net/publication/279706380
[27] Santos PAG, Figueiredo CA, Lourenço PML, Barroso JG, Pedro LG, Oliveira MM et al., and Hairy root cultures of Anethum graveolens (dill): Establishment, growth, timecourse study of their essential oil and its comparison with parent plant oils, Biotechnology Letters, 2002; 24:1031– 1036.
[28] Merazi Y, Hammadi K. Bacterial activity of some medicinal plants used as an avian therapy in Algeria. Am. J. Microbiol. Biotechnol. 2017; 4(6): 108-114
http://www.aascit.org/journal/ajmb.
[29] Borugă O, Jianu C, Mişcă C, Goleţ I, Gruia AT, Horhat FG. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity, J. Med. Life, 2014; 7(3): 56-60.
[30] Ivanovic J, Misic D, Zizovic I, Ristic M. In vitro control of multiplication of some food-associated bacteria by thyme, rosemary and sage isolates. Food Control, 2012; 25(1): 110-116.
https://doi.org/10.1016/j.foodcont.2011.10.019.
[31]Silva N, Alves S, Gonçalves A, Amaral JS, Poeta P. Antimicrobial activity of essential oils from Mediterranean aromatic plants against several foodborne and spoilage bacteria, Food Science and Technology International, 2013; 19(6): 503-510.
https://doi.org/10.1177/1082013212442198.
[32] Burt S. Essential oils: their antibacterial properties andpotential applications in foods – a review, Int J Food Microbiol, 2004; 94:233–253.
[33] Holley RA, Patel D. Improvement in shelf-life and safety ofperishable foods by plant essential oils and smokeantimicrobials, Food Microbio, 2005;22:273–292.
https://doi.org/10.1016/j.fm.2004.08.006.
[34] Souza EL, Lima EO, Naraim N. Especiarias: uma alternativapara o controle da qualidade sanitária e de vida útil dealimentos, frente às perspectivas da indústria alimentícia, Hig Aliment, 2003; 17:38–42.
[35] Bassolé IH, Lamien-Meda A, Bayala B, Tirogo S, Franz C, Novak J, Nebié RC, Dicko MH. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination,Molecules, 2010; 15 (11): 7825-7839. https://doi.org/10.3390/molecules15117825.
[36] Soković M, Glamočlija J, Marin PD, Brkić D, van Griensven LJLD. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model, Molecules, 2010; 15 (11): 7532-7546.
https://doi.org/10.3390/molecules15117532.
[37] G ómez-Estaca J, López de Lacey A, López-Caballero ME, Gómez-Guillén MC, Montero P. Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation, Food Microbiol, 2010; 27 (7): 889-896.