|
After Successful of Tocilizumab Plus Micronutrients in The Treatment of COVID19 Patients; Cosentyx May Be Recommended
Hewida H. Fadel1*, Mohamed Abd El-Rahman Ahmed2, Kareem MahamoudGharbeya3, Mohammed Ahmed Khamis Mohamed4, Reda Almiry5
|
|
1Department of Medical Laboratory Technology, Faculty of Allied Medical Science, Pharos University, Alexandria, Egypt. 2Department of Clinical Pathology, Military Medical Academy, Alexandria Armed Forces Hospital, Egypt. 3Critical Care Consultant at Alexandria Armed Forces Hospital, Egypt. 4Pulmonary Consultant at Alexandria Armed Forces Hospital, Egypt. 5Molecular and diagnostic microbiology, Molecular microbiologist consultant at Alex Armed Forces Hospital, Egypt. |
ABSTRACT
By 23rd August 2020, 23,057,288 confirmed cases with SARS-CoV-2 that causes 800,906 deaths worldwide. The severe illness and high mortality are attributed to the dysregulation of hematopoiesis accompanied by cytokines storm. The severe illness and high mortality rate confirm the failure of the conventional strategy in the treatment of COVID19 patients. Also, lack of a specific vaccine or therapies target for SARS-CoV-2 infection, the drug repositioning in the treatment of COVID19 patients is the only opportunity to face this pandemic. Thus, we highlight the implication of a novel strategy of treatment based on the inhibition of IL-6, the major player in a cytokine storm, using Tocilizumab, in combination with micronutrients including Zinc, Selenium, vitamin C, and Glutathione. Application of Tocilizumab in infectious diseases is considered as a new treatment protocol since its usual uses are in autoimmune diseases. In the present study, we analyze the data for COVID19 patients who showed cytokine storm due to elevated level of IL-6 and suffered from severe illness, fever, ARDS, and transferred to ICU in Armed Forces Hospital. We found that Tocilizumab plus micronutrients improves the clinical outcomes in these critical cases of COVID19 patients. Statistical analyses of biochemical parameters including complete blood count to evaluate the efficacy of this combined therapy on counteracting the effect of SARS-CoV-2 on the differential of blood cell ratios, pro-inflammatory parameters (CRP, D-dimer, and ferritin), liver, cardiac, and kidney function were carried out. Finally, based on the success of Tocilizumab, an IL-6 inhibitor, in the treatment of COVID19 patients, we recommend using Cosentyx that is an inhibitor of IL-17, a partner of IL-6 in the inflammation process.
Key Words: SARS-CoV-2, COVID19, Interleukin inhibitors, IL-6, Tocilizumab, micronutrients, IL-17, Cosentyx, Pneumonia.
INTRODUCTION
To date, COVID19 prevalence still progresses rapidly and has attacked many people and caused millions of deaths worldwide [1]. In the current pandemic, strong evidence revealed that the severity and high mortality among COVID19 patients are attributed to modulation of the hematopoiesis, shifting the cell differentiation towards certain elements causing disorders in both blood cell ratios and vigorous activation of the immune system and cytokines storm [2-4]. Many aspects support this point of view, firstly; systemic spread of the virus with a wide spectrum range of symptoms resulting in severe complications that are extended beyond acute respiratory distress syndrome (ARDS) to multiple organ dysfunction syndromes (MODS) including kidney, liver disorders, cardiac injury, thrombosis and arrhythmia [5-7], secondly; thrombosis complications play a major role in all of these organ failures and cause death. In this aspect, it is well known that thrombosis formation in the vessels due to higher levels of D-dimer and fibrinogen is associated with high mortality. In addition, scientists reported additional non-specific symptoms on the skin such as Pityriasisrosea, exanthematous rash, urticaria, chickenpox like vesicles, petechiae. Also, neurologic manifestations and acute hemorrhagic edema were reported in a patient infected with SARS-CoV-2 [8, 9]. All of these confirm that SARS-CoV-2 infectivity extends beyond the influenza virus. Symptoms of influenza virus infection may last for 7–10 days and are self-limiting due to the induction of a protective immune response [10]. Years ago, scientific research focused on understanding the immune response to viral infection to induce protective immunity in populations at the extremes of age and in immune-compromised subjects, the most liable targets for infectious induced-severe illness and high mortality [11]. Subsequently, a novel treatment strategy based on immunomodulatory therapies to counteract COVID19-induced cytokine storm is widely used and improving the clinical outcomes [12]. Therefore, we focus on a new strategy in the treatment of COVID19 patients who did not respond to the conventional treatment protocol, particularly elderly ones who suffer from diabetes, cardiac disease, and hypertension. Follow up patients and assessment of biochemical markers gave us a profile about the effect of IL-6 on blood parameterssuch as Monocytes/lymphocytes ratio (MLR), Neutrophiles/lymphocytes (NLR), platelets/lymphocytes ratio (PLR), pro-inflammatory markers (CRP, ferritin, and D-dimer) as well as their impact on blood homeostasis by assessment of electrolytes (Na/K concentrations), in addition to, biomarkers of kidney and liver function and clinical examination of lung function by CT before and after treatment as well as the assessment of arterial oxygen saturation. Among many cytokines, interleukin 6 (IL-6) has the main role as it has pro- and anti-inflammatory effects, thermoregulation, bone remodeling, and differentiation of fibroblasts cells in patients with pulmonary fibrosis [13]. An old strategy to reduce IL-6 in chronic inflammatory diseases is based on Hydroxy-chloroquine. A recent clinical trial proved that Azithromycin and Doxycycline inhibit viral replication of SARS-CoV-2 via inhibition of IL-6 production [14, 15]. Advanced strategy based on blockade of IL-6 signal cascade by using Tocilizumab (Actemra), a monoclonal antibody targeting the IL-6 receptor, may mitigate the effects of cytokines released in response to the virus and limit lung damage in COVID19 patients with severe disease. Ongoing clinical trials emerge the success of Tocilizumab in relief of the severe illness in patients with COVID-19 [16]. For optimizing the therapeutic use of interleukin inhibitors, a combination with micronutrients such as Zinc, Selenium, Glutathione (GSH), and vitamins C & D is very important [17]. This retrospective study analyzes the data of COVID19 patients complicated with ARDS who were treated with Tocilizumab in the intensive care unit (ICU) of Forced Armed Hospital.
MATERIALS AND METHODS
Among 245 patients (178 males and 67 females) admitted to Armed Forces Hospital from 12th April to 30th June 2020 were diagnosed with COVID19 based on positive PCR for SARS-CoV-2 and clinical examination. The usual protocol for COVID19 patients includes Plaquenil (200 mg), Azithromycin (500mg), vitamins and minerals (vitamin C&D, Zinc Magnesium, Selenium), Acetylcysteine (20% nebulizer), lactoferrin (250 mg). Among them, twenty-three critical cases who admitted ICU based on CT scan and the clinical manifestation showed an elevated level of IL-6 (mean 181.4 ng/ml ± 14.1). They suffered from fever, dry cough, dyspnea, pneumonia, and hypoxia, lowering of blood oxygen saturation (SpO2) to a level of 75% room air, they put on 10L oxygen. Patients were evaluated for the possibility of cytokines storm and Tocilizumab was initiated. Eleven patients needed 400 mg Tocilizumab (intravenous) once while twelve patients needed 400 mg twice (12 hrs). They had provided written informed consent to publish their cases details. Serum IL-6 level was assessed once as a baseline to take a decision of therapy. The data were collected manually from patient’s charts in Archive, medical laboratory, and medical radiological imaging Departments. Laboratory values for hematological analysis (complete blood count and pro-inflammatory biomarkers; CRP, ferritin, D-dimer, and IL-6), liver function (ALT and AST), kidney function (Urea, Creatinine) and electrolytes (Sodium, potassium) of all patients were obtained. Also, the demographic and clinic pathologic data were obtained including: age, sex, clinical history, and smoking. The values were compared at the time of intensive care unit (ICU) admission and time of discharge from ICU.The study was approved by the Ethics Committee of the Medical Research Institute, Alexandria University, Egypt (IORG 0008812).
Statistical Analysis of the Data
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). Qualitative data were described using the number and percent. The Kolmogorov-Smirnov test was used to verify the normality of distribution. Quantitative data were described using range (minimum and maximum), mean, standard deviation, median, and interquartile range (IQR). The significance of the obtained results was judged at the 5% level. The Paired t-test was used and P ≤ 0.05 was considered as statistically significant.
RESULTS
Demographic characteristics
Among the thirty-two patients were selected who were admitted to ICU with the age range of 32-75 years, including 14 males and 9 females. Of them, 34.7% were smokers. All of them showed pneumonia (100%) and 82.6% showed dyspnea, 73.9% had a fever, 91.3% had dry coughs. The clinical history of them showed that 82.6% were diabetic, 47.8% had CVD, 39.1% had hypertension, and 17.3% had kidney diseases as shown in Table 1.
Impact of Tocilizumab plus micronutrients on clinical manifestations
In the present study, most patients admitted ICU based on CT where they showed bilateral ground-glass opacities involving both lungs, CORAD-6 as shown in Figure 1. Within 7- 10 days in ICU, among twenty-three COVID19 patients who underwent Tocilizumab plus micronutrients, nineteen patients showed a significant increase in SpO2:97%, improved lung function, and fever and pneumonia relief, thus, they discharged from ICU while four patients died.
Effect of Tocilizumab plus micronutrients on hematopoiesis and pro-inflammatory markers
In the present study, in response to Tocilizumab therapy, the absolute number of monocytes and neutrophils were decreased significantly (p=0.001 and p˂0.001, respectively) while the absolute number of platelets increased significantly (p˂0.001). However, there was no significant change in lymphocytes. The present data showed a significant increase in PLR in response to Tocilizumab (p˂0.001), while MLR and NLR did not show any significant difference before and after therapy. Also, we noticed that both CRP and ferritin significantly decreased (p˂0.001) in response to Tocilizumab plus micronutrients, while D-dimer significantly increased (p˂0.001), as shown in Table 2.
Effect of Tocilizumab plus micronutrients on liver and kidney functions
Significant elevation of ALT and AST levels was noticed in response to Tocilizumab plus micronutrients (p<0.002 and p=0.011, respectively). Significant decreases in the urea and creatinine levels were noticed in response to Tocilizumab plus micronutrients (P <0.001). A significant decrease in potassium levels was noticed in response to Tocilizumab plus micronutrients (p<0.001) while sodium level did not show any significant difference as shown in Table 2.
Table 1. Demographic and clinical features of COVID19 patients upon admission ICU
|
Characteristics |
Number of COVID19 patients n=23 (%) |
|
Sex |
|
|
Male |
14 (60.8%) |
|
Female |
9 (39.1%) |
|
Age |
32-75 |
|
Pneumonia |
23 (100%) |
|
Cardiovascular diseases |
11(47.8%) |
|
Diabetes |
19(82.6%) |
|
Hypertension |
9(39.1%) |
|
Kidney disease |
4(17.3%) |
|
Dry cough |
21(91.3%) |
|
Dyspnea |
19(82.6%) |
|
Fever |
17(73.9%) |
|
Diarrhea |
8(34.7%) |
|
Smoking |
8(34.7%) |
Table 2. Hematological and biochemical analysis of blood samples from COVID19 patients before and after Tocilizumab.
|
Laboratory findings (units ) |
Before Tocilizumab (Mean ± SD.) |
After Tocilizumab (Mean ± SD.) |
P |
|
TLC (×109/L) |
12.9 ± 0.87 |
8 ± 1.03 |
<0.001* |
|
Lymphocytes (×109/L) |
1.7 ± 0.73 |
1.2 ± 0.37 |
0.187 |
|
Monocytes (×109/L) |
0.98 ± 0.16 |
0.81 ± 0.10 |
=0.001* |
|
Neutrophiles (×109/L) |
7.5 ± 0.95 |
5.6 ± 1.1 |
<0.001* |
|
Platelets (×109/L) |
504.2 ± 47.4 |
715.7 ± 91.1 |
<0.001* |
|
MLR |
0.65 ± 0.23 |
0.71 ± 0.21 |
0.514 |
|
NLR |
5.1 ± 2.2 |
5 ± 1.6 |
0.097 |
|
PLR |
339 ± 132.7 |
605.1 ± 201.8 |
<0.001* |
|
D-dimer(mg/ml) |
1.1 ± 0.33 |
2 ± 0.35 |
<0.001* |
|
Ferritin(ng/ml) |
1919.2 ± 30.9 |
806.3 ± 63 |
<0.001* |
|
CRP (mg/L) |
24.6 ± 25.8 |
7.9 ± 2.9 |
<0.001* |
|
ALT (IU/L) |
45.7 ± 9.7 |
57 ± 11.1 |
=0.002* |
|
AST(IU/L) |
64.2 ± 8 |
71.3 ± 6.6 |
=0.011* |
|
Urea (mg/dl) |
59 ± 5.9 |
46.6 ± 5.5 |
<0.001* |
|
Creatinine(mg/dL) |
1.5 ± 0.24 |
0.96 ± 0.15 |
<0.001* |
|
Sodium(mmol/L) |
128.6 ± 6.7 |
125.4 ± 2.2 |
=0.126 |
|
Potassium (mmol/L) |
3.6 ± 0.11 |
3.4 ± 0.12 |
<0.001* |
P: P-value for comparing between the studied periods
*: Statistically significant at P ≤ 0.05
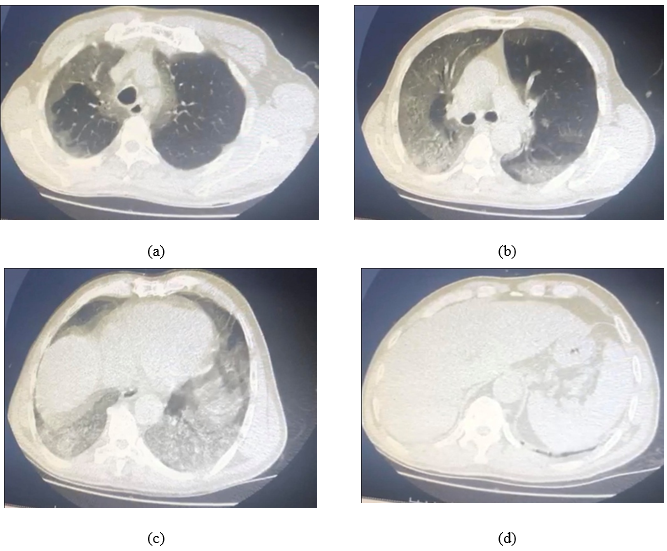
Fig. 1: CT for a case who was confirmed with COVID19 by PCR result shows CO-RADS: 6 a) bilateral peripheral multifocal ground-glass lesions in the upper lobe of the lung, b) bilateral consolidative peripheral lesions more prominent on the middle lobe of the right lung, c) bilateral diffuse consolidative lesions allover both lungs, c) ground-glass appearance of the left lower lobe of the lung
.
DISCUSSION
In the present study, COVID19 patients who had been admitted to ICU had a history of diabetes, hypertension, and cardiovascular diseases (CVD). Additionally, they did not respond to the conventional treatment protocol including Plaquenil (200mg), Azithromycin (500mg), a combination of vitamins and minerals (vit-C, vit-D, Zinc, Magnesium, Selenium), Acetylcysteine (20% nebulizer), and lactoferrin (250 mg). Symptoms of COVID19 progressed rapidly and manifested as persistent fever, pneumonia, and sudden multiple organs’ dysfunction including liver and kidney accompanied by the significant elevation of pro-inflammatory markers such as IL-6, CRP, ferritin, and D-dimer.
The present data revealed that before the initiation of Tocilizumab therapy, IL-6, CRP, ferritin, and D-dimer elevated significantly and accompanied by the severe illness in COVID19 patients who were transferred to ICU. Consistent with our results, a recent study indicated that IL-6, CRP, ferritin, and D-dimer are positively correlated with the severity of COVID19. In addition, low levels of serum iron and hyperferritinemia are common in humans exposed to high pathogen loads as a possible mechanism to protect the host during an active infection, limiting the availability of iron to pathogens [18, 19]. After Tocilizumab therapy plus micronutrients, a significant decrease in CRP and ferritin was observed while D-dimer significantly increased. An explanation of the continuous increase of D-dimer may be attributed to the observed liver disorder where SGOT and SGPT elevated significantly since liver is responsible for the clearance of D-dimer. Consistent with our results, a recent study revealed that D-dimer is strongly associated with liver disease [20].
Failure of conventional therapies in this study proves the importance of the integration of new therapies in the treatment of COVID19 including immunomodulatory therapies such as IL-6 inhibitor (Tocilizumab). Our data indicate an improvement of the clinical outcomes in COVID19 patients who were treated with Tocilizumab plus micronutrients after 3-5 days of initiation therapy where fever and pneumonia were relieved as well as pro-inflammatory markers significantly decreased. An ongoing clinical trial revealed that IL-6 inhibitor (Tocilizumab) improves the clinical outcomes in patients with COVID-19 who display elements of cytokine storm with markedly elevated interleukin-6 level, which was in line with our results [16, 21]. Also, the efficacy of Tocilizumab in the treatment of rheumatoid arthritis (RA), macrophage activation syndrome (MAS), and coronary diseases with severe complication of Kawasaki disease (KD) were reported [22, 23]. This may be helpful in the treatment of rare cases reported in COVID19 pandemic in children with KD.
As noticed in COVID19 patients, it has been demonstrated that viral infection affects the hematopoiesis and shifts cell differentiation towards certain elements. As mentioned above, disturbance in the differentiation of immune cells as well as their ratio results in the severity of illness in COVID19 patients. In the present study, a significant increase of PLR in response to Tocilizumab plus micronutrients including Zinc was associated with improvement of the clinical outcomes. This is consistent with a recent study, which revealed that increasing PLR is associated with surviving of COVID19 patients. Particularly, Zinc is an integral part of Zinc finger proteins (ZFPs) that play an important role in the proliferation and differentiation of hematopoietic precursor cells [24]. Also, Vit-C and Zinc have pivotal roles in maintaining the three main immunoreactive clusters; physical barriers, innate and adaptive immunity. Also, Zinc is an integral element in MMPs that protects against infectious diseases via controlling the immune responses and signaling by regulating the activity of effector proteins [25, 26]. Moreover, Zinc decreases cytokine production (IL-6, IL-2, IL-10) [27]. Previous studies have shown that zinc and vit-D maintain the Th1/Th2 ratio [28]. Also, a recent study revealed that GSH relieves pneumonia in COVID19 patients via inhibition of TNF-α-induced NF-kappaB activation in mononuclear cells including monocytes and lymphocytes [29], subsequently, reduces the cytokine storm as shown in current cases who showed improvement in clinical outcomes.
Although Gómez-Rial et al., indicated the implication of SARS-CoV-2 on monocytes–macrophage differentiation, we did not notice any significant change in MLR and NLR in response to Tocilizumab [30]. However, another study revealed that in response to viral infection, IFNγ enhances myelopoiesis; production of monocytes and neutrophils, which play crucial roles in viral clearance [31]. Also, many studies revealed the association between SARS-CoV-2 infection and thrombosis induced-death. This may explain the failure of using corticosteroids alone in patients infected with a viral infection and SARS-CoV-2, where they may increase the risk of sepsis, allergies, or spinal diseases and venous thromboembolism that may progress to fatal outcomes [32]. It has been demonstrated that thromboembolism may be associated with elevation of pro-inflammatory markers including IL-6, D-dimer, and CRP in viral infected patients [33].
In the present study, severe pain, fever, and fatigue were reported in COVID19 patients. Of noted, progressive muscle pain, and weakness were noticed in patients with viral infection due to myositis and bone remodeling. This fatigue was progress prior to the manifestation of fever, sore throat, and myalgias in patients with viral infection [34,35]. Also, IL-6 is associated with bone turnover in inflammatory diseases [36]. In the present study, hypoxia was reported in all patients who suffered from the elevation of IL-6-induced cytokines storm. Of course, hypoxia is a major stimulator of bone resorption, inducing osteoclastogenesis, which is later followed by osteoblastogenesis [37, 38]. The noticed acidosis resulting from hyponatremia resulting from hyperventilation may have an impact on feeling pain and osteoporosis where metabolic or local acidosis can induce osteoclast activation and bone loss [39]. Also, acidosis may result from hypoxia and it is well known that bone resorption is activated under low pH in order to release phosphates and restore acid-base equilibrium in extracellular fluid. However, Okito et al. recently observed that under acidic conditions osteoblasts tend to change their phenotype from osteogenic to osteoclastogenic [40]. It is well established that infection with SARS-CoV-2 induces osteoporosis via increased urinary calcium excretion [41].
Elevated levels of urea and creatinine were observed before initiation of Tocilizumab therapy while SGOT and SGPT showed insignificant change. After therapy, kidney function was improved while liver disorder was noticed since SGOT and SGPT were elevated significantly. Also, we noticed a significant decrease in sodium and potassium levels after therapy. It is consistent with many studies that revealed an association between hyponatremia and hypokalemia with immunotherapies [42]. However, significantly elevated levels of ALT and AST indicated liver disorder in response to Tocilizumab. It is well established the hepatotoxicity of immunotherapies and sometimes if liver toxicity records showed grades 3 or 4, discontinuation was considered and treatment with prednisolone was recommended [43].
From previous, we hypothesized the following:
As noticed in global and our clinical trials, the efficacy of Tocilizumab in relief the cytokine storm in COVID19 patients may open the field to apply more interleukins inhibitors such as Cosentyx, an IL-17 inhibitor. Our hypothesis based on; firstly, the pathological role of IL-17 in pulmonary inflammation and asthma[44], subsequently its inhibition by Cosentyx may add benefits in the treatment of COVID19 patients; secondly, the strong correlation between IL-6 and Il-17 where both regulate the expression of each other and pro-inflammatory proteins such as CRP [45]; thirdly, no evidence reports any increase in the rate of COVID19 incidence or mortality in autoimmune patients who have been already treated with Cosentyx. Thus, our ongoing research study is carried out to assess the IL-17 level in COVID19 patients as a first step to begin a clinical trial on Cosentyx.
CONCLUSION
Combination therapy of Tocilizumab plus micronutrients including Zinc, Selenium, vitamin C & D, and cysteine prodrug may improve the lung function in COVID19 via regulation of hematopoiesis and maintenance the immune balance.
Conflicts of Interest
The authors declare no conflict of interest.
Authors’ Funding
No funding from any organization.
Author Contributions
H. F. analyzed, interpreted the biochemical data, and wrote the manuscript, R.E. laboratory analysis, K.F.ICU management, M.K. treatment and follow up patients outside ICU, M.A. revised the manuscript and supervised the research.
Abbreviations
COVID-19: Coronavirus disease 2019; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; ARDS: respiratory distress syndrome; ICU: Intensive care unit; CT: computerized tomography; CRP: C-reactive proteins; MLR: Monocytes/lymphocytes ratio; PLR: platelets/lymphocytes ratio; MODS: multiple organ dysfunction syndromes; CVD: cardiovascular diseases; GSH: glutathione; CRS: Cytokine release syndrome; RA: rheumatoid arthritis; IL-6: Interleukin-6; IL-17: Interleukin-17; MAS:Macrophage activation syndrome; KD: Kawasaki disease; ZFPs: Zinc finger proteins; MMPs:matrixmetalloproteinases; TLC: total leukocytes; ALT: Alanine transaminase; AST: Aspartic transaminase; INR: international normalized ratio
ACKNOWLEDGMENT
We thank the Armed Forces to provide patients with Tocilizumab as a free donation. We also thank Mabaret El-Asafra Lab to perform IL-6 analysis by ELISA as soon as we sent it.
ORCID
Hewida H. Fadel, https://orcid.org/0000-0002-6696-0405
LinkedIn Account (URL)
Implications:
Tocilizumab, an IL-6 inhibitor, plus micronutrients including Zinc, Selenium, vitamin C & D, and Glutathione may be a novel treatment protocol for COVID19 patients. Cosentyx, an IL-17 inhibitor, may be a novel therapy in the treatment of COVID19 patients who suffered from severe illness due to cytokines storm.
REFERENCES
Monin L, Gaffen SL. Interleukin 17 Family Cytokines: Signaling Mechanisms, Biological Activities, and Therapeutic Implications. Cold Spring HarbPerspectBiol 2018; 10(4):a028522.

