



|
A Review on Chiral Stationary Phases for Separation of Chiral Drugs
Celina Nazareth*, Sanelly Pereira |
|
Department of Pharmaceutical Chemistry, PES’s Rajaram and Tarabai Bandekar College of Pharmacy, Farmagudi, Goa, India-403401. |
ABSTRACT
The resolution of chiral drugs is important in pharmaceutical analysis. The enantiomers of a chiral drug having identical physical and chemical properties in an achiral environment can be distinguished in a chiral environment provided that there are suitable interactions with a chiral selector. The enantiomers of a chiral drug can act differently with one enantiomer exhibiting pharmacological activity and the other exhibiting undesirable toxic effects. It is therefore critical to separate and analyze racemic drugs. The United States Food and Drug Administration guidelines, therefore, make it mandatory to market only the active enantiomer of chiral drugs. The availability of innovative chiral stationary phases makes the identification of enantiomers faster and effective. This review describes the different chiral stationary phases based on Pirkle type, protein, cyclodextrin, polysaccharide, ligand exchange, macrocyclic antibiotics and chiral crown ethers used in chiral chromatography. The various mechanisms involved in the chiral recognition of enantiomers are summarized.
Key Words: Chiral chromatography, chiral stationary phase, racemic drugs, enantiomer separation.
INTRODUCTION
Enantiomers [1] are non-superimposable mirror images of a chiral molecule having similar physical and chemical properties. A large number of drugs, such as β-blockers (metoprolol) [2], antibiotics (D-cycloserin) [3], statins, anticoagulants, anti-inflammatory agents are marketed as racemic mixtures (racemate) having equimolar amounts of R and S enantiomers of a chiral molecule. These enantiomers present a difference in pharmacological activity or efficacy. Each enantiomer of a chiral molecule can act uniquely, exhibiting different plasma concentration, bioavailability, and toxicology [4]. Therefore, it is of paramount significance to analyze drugs with a chiral center for different bioactivities and to determine the concentration of each enantiomer in biological fluids. However, the similarity in the physical and chemical properties creates a challenge in the separation of enantiomers [5, 6]. Separations can, therefore, be achieved by reacting the sample with a chiral compound or a chiral stationary phase to form diastereomers with different physical and chemical properties. The United States Food and Drug Administration (USFDA) requires the evaluation of enantiomers as well as the racemic mixtures of a chiral drug before clinical use.
Enantiomeric separation can be accomplished by chiral chromatography. Chiral chromatography includes the use of gas chromatography (GC), supercritical fluid chromatography (SFC), and high-performance liquid chromatography (HPLC). However, chiral HPLC is the most widely used of these methods [4, 7]. Enantioselective determination can also be carried out using hyphenated techniques (LC-MS/MS) [2, 8, 9], micellar electrokinetic chromatography [10, 11] and capillary electrophoresis (CE), the latter being a powerful alternative to chromatographic techniques. Several chiral separation principles successfully applied in HPLC have been transferred to CE due to the advantages of CE employing small amounts of chiral selector and solvents [12]. This article provides an insight into the various chiral stationary phases used in chiral chromatography along with the mechanism of separation and applications.
Need for chiral separation:
The primary goal of chiral separation is to overcome the challenge of similarity in the physical and chemical properties of enantiomers by forming diastereomers which possess different physical and chemical properties. Pharmacokinetic and chiral inversion studies of enantiomers become necessary as pharmacokinetics of enantiomers may be stereoselective and chiral inversions may occur in-vivo or in-vitro [8].
Principle of chiral chromatography:
The ability of the CSP to interact differently with two enantiomers, leading to their HPLC separation is known as chiral recognition. Chiral recognition depends on different interactions such as hydrogen bonding, π-π interaction, dipole stacking, inclusion complexation, steric bulk between the analyte and the CSP, hydrophobic and electrostatic interaction, charge-transfer interactions, ionic interactions to form transient-diastereomeric complexes.
Chiral separations by HPLC can be achieved by:
I) Direct Separations
The direct method of separation involves the actual chromatographic separation of molecules that are enantiomerically related to each other [7]. Here, the stationary phase consists of a chiral substance chemically bonded to stationary phase support to form a chiral stationary phase (CSP) [7]. Separation involves an interaction between the CSP and the racemic drugs to form diastereomeric complexes. One of these complexes will have a stronger binding strength than the other, resulting in different retention times for the enantiomeric pair.
Another type of direct method uses a chiral mobile-phase additive (CMPA), which forms a transient diastereomeric complex with the analyte. The resolution of these diastereomeric complexes is then possible by HPLC [7]. This approach is not used due to difficulties such as the requirement for a continuous supply of CMPA, difficulties in detection and poorly shaped peaks. The chiral mobile phase approach represents a simple and flexible alternative, which is, however, not always applicable [13]. The mobile phase which contains the chiral selector cannot be reused.
II) Indirect Separations
The indirect method is based on chiral derivatization, which involves the reaction of the enantiomers with a chiral derivatizing agent (CAD) to form diastereomeric derivatives differing in their physical and chemical properties. These diastereomers are then separated on an achiral stationary phase. Chiral derivatization reagents used in indirect methods are chiral Marfey’s reagent (1-fluoro-2,4-dinitrophenyl-5-L-alanine amide), Sanger’s reagent (1-Fluoro-2,4-dinitrobenzene), and o-phthaldehyde with N-acetyl-L-Cysteine. This approach circumvents the need for expensive columns with chiral stationary phases and is more flexible; however, the disadvantage of this approach is the additional step which can involve undesirable side reactions, the formation of decomposition products and racemization. Additionally, the chiral derivatization reagent should possess high enantiomeric purity and have derivatizable groups in the analyte [13].
Choice of a suitable method is governed by several factors such as (a) required assay sensitivity, (b) ready availability, purity and stability of chiral derivatizing agent, (c) efficiency and ease of derivatization, (d) suitable chiral stationary phase, (e) simple and easy optimization of chromatographic conditions and (f) overall analysis time [2].
TYPES OF CHIRAL STATIONARY PHASES:
Among these CSPs, polysaccharides and macrocyclic antibiotics based CSPs are very important as they have achieved a great reputation in the field of chiral resolution [14].
Pirkle type (Donor-Acceptor Columns)
Pirkle type chiral stationary phases are also called as brush type. Separation on these CSPs is based on a three-point attachment between the solute and the CSP. These interactions may be attractive or repulsive in nature. Pirkle columns discriminate enantiomers by binding of one enantiomer with the chiral stationary phase, thereby forming a diastereomeric complex through π-π bonding, hydrogen bonding, steric interactions, and/or dipole stacking [7]. Pirkle CSP can be categorized into three classes:
The most widely used Pirkle chiral stationary phase following commercial introduction has been 3,5-dinitrobenzoylphenylglycine (DNBPG) CSP. This type of chiral stationary phase is called π-electron acceptor: effectively resolves enantiomers containing a π-electron donor such as aromatic enantiomers. The π-π (intermolecular) interactions between the aromatic ring of a solute and CSP are important factors in enantioselectivity with the CSP. The DNBPG CSP also contains two acidic hydrogen and two basic carbonyl groups which can form a hydrogen bond with analytes containing functional groups such as amides, amines or hydroxyls. The second type of Pirkle stationary phase contains a π-electron-donating moiety which is used for the separation of drugs containing amines, alcohol, carboxylic acids, thiols, etc. A naphthalene ring is a strong π-electron donor. The third kind of column is the hybrid containing both π-electron acceptor and π-electron donor moiety e.g. the Whelk-O 1. This bears both π-acidic and π-basic interaction sites that are capable of resolving enantiomers containing either π-acid or π-base. Figures 1, 2 and 3 show the chemical structures of the π-electron acceptor, π-electron donor, and π- electron donor acceptor CSP respectively [7]. Table 1 lists some commercially available pirkle type columns.
Table 1: Some commercially available pirkle-type columns
|
Trade Name |
Bonding |
Class |
|
β-Gem 1 |
N-3,5-dinitrobenzoyl-3-amino-3phenyl-2-(1,1-dimethylethyl)-propanoate |
π-electron acceptor |
|
α-Burke 2 |
Dimethyl N-3,5-dinitro-benzoyl-α-amino-2,2-dimethyl-4-pentyl phosphonate |
π-electron acceptor |
|
Leucine |
3,5-Dinitrobenzoyl leucine |
π-electron acceptor |
|
Whelk-O 1 |
1-(3,5-dinitrobenzamido)-1,2,3,4- tetrahydrophenanthrene |
π-electron acceptor/donor |
|
Phenylglycine |
3,5-Dinitrobenzoyl phenylglycine |
π-electron acceptor |
|
ULMO |
3,5-Dinitrobenzoyl derivative of diphenylethylenediamine |
π-electron acceptor/donor |
|
DACH-DNB |
3,5-Dinitrobenzoyl derivative of 1,2-diaminocyclohexane |
π-electron acceptor |
|
Pirkle 1-J |
3-(3,5-Dinitrobenzamido)-4-phenyl-β-lactam |
π-electron acceptor |
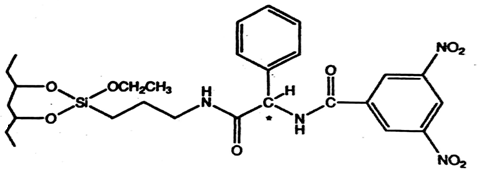
Fig. 1. Chemical structure of 3,5-dinitrobenzoyl phenylglycine (3,5-DNBPG) CSP, a π-electron acceptor CSP [7]
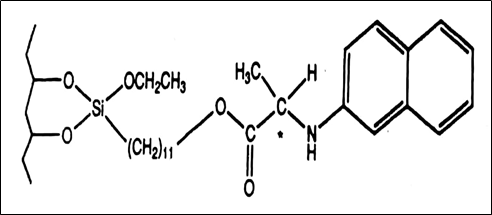
Fig. 2. Chemical structure of naphtylalanine CSP, a π-electron donor CSP [7]
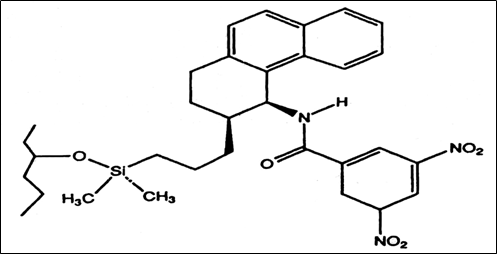
Fig.3. Chemical structure of the Whelk-O 1 CSP, a π-electron donor-acceptor CSP [7]
Protein-based chiral stationary phases
While many protein chiral stationary phases have been proposed, six materials with somewhat different characteristics now have been commercialized in columns for chiral separations: bovine and human serum albumin, ɑ1- acid glycoprotein (orosomucoid), ovomucoid, cellobiohydrolase (cellulase), and pepsin [7]. The mechanism of chiral interaction between the protein and the analyte involves hydrophobic and electrostatic interactions, hydrogen bonding and charge-transfer interactions may also contribute to chiral recognition. Hydrophobic interactions between the protein and the analyte are affected by percent organic in the mobile phase. As the organic content increases, retention on protein-based columns decreases. Table 2 shows some commercially available protein-based columns.
Table 2: Some commercially available protein-based columns
|
Trade Name |
Chiral Selector |
|
Chiral-AGP |
ɑ1- acid glycoprotein (AGP) |
|
Chiral HAS |
Human serum albumin (HSA) |
|
Chiral CBH |
Cellobiohydrolase (CBH) |
|
Ultron ES-OVM |
Ovomucoid |
|
Ultron ES-Pepsin |
Pepsin |
|
Resolvosil BSA-7 |
Bovine serum albumin |
The chiral AGP column is a glycoprotein containing 183 amino acids and carbohydrate moiety in the form of N-linked glycans, having an isoelectric point of 2.7–3.8. The carbohydrate moiety is believed to be involved in the binding of compounds. The enantioselective properties of chiral-AGP may be affected by several chromatographic parameters such as the nature of buffer, pH value of the buffer, organic modifier (nature and percentage), buffer concentration, presence of charged additives in the mobile phase, and the temperature [15].
Polysaccharide chiral stationary phases
The naturally occurring polysaccharide form the basis for an important group of columns designed for chiral separations [7]. The main polysaccharides are cellulose, amylose, chitosan, dextran, xylan, curdlan, and inulin [16]. However, due to their low-resolution capacities and handling problems, they cannot be used as such and hence, their derivatives have been synthesized [17].
Polysaccharide-based columns have a high loading capacity and can be used for the separation of a wide range of compounds. Microcrystalline cellulose triacetate (MCT), a product of the heterogenous acetylation of microcrystalline cellulose particles has been one of the first useful column packings developed for chiral HPLC separation [7]. These columns have lower efficiency and therefore are not widely used.
Derivatives of cellulose and amylose exhibit excellent properties for the separation of chiral molecules. The former has a rigid linear structure with a ß-(1,4)-D-glucose linkage while the latter has a helical structure with an ɑ-(1,4)-D-glucose linkage. The glucose units have a chair conformation with 2-OH, 3-OH, and 5-CH2OH groups all in the equatorial position [18, 19]. The different glucose linkages give rise to the different higher-order structures of these polymers in which the polymers are held by intramolecular and intermolecular hydrogen bonds [20]. Figure 4 shows the chemical structure of cellulose and amylose polymers [18]. The three-dimensional structures of amylose and cellulose polymers are depicted in figure 5 [14].
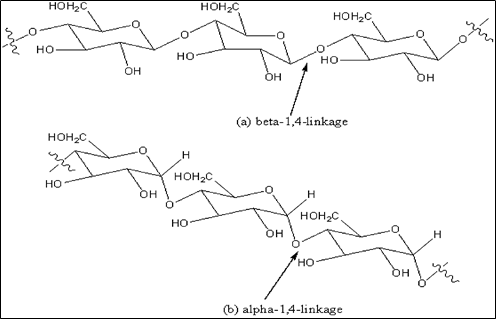
Fig.4. Chemical structures of (a) cellulose and (b) amylose polymers [18]
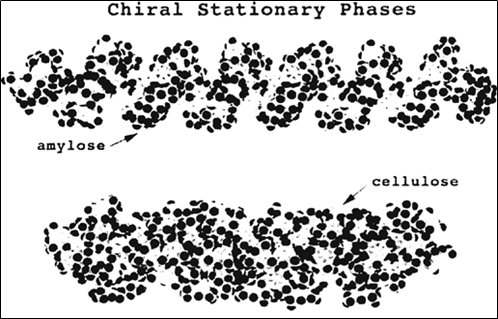
Fig.5. Three-dimensional structure of amylose and cellulose chiral stationary phase [14]
Polysaccharide-based chiral stationary phases have a wide application in LC-MS due to their high separation efficiency, selectivity, sensitivity and reproducibility under normal and reversed-phase conditions as well as their broad applicability for structurally diversified compounds [21]. The mechanism of chiral interaction on the polysaccharide-based chiral stationary phase has not yet been elucidated. However, the following interactions are believed to play a role in the retention:
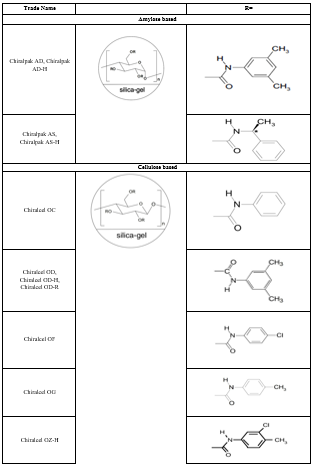
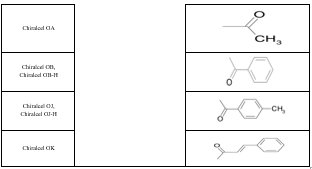
Some of the factors that play an important role in the retention process include the functionality used to derivatize the polysaccharide, the average molecular weight and molecular weight distribution of the polymer, the solvent used to deposit the polymer on the support and the nature of the support itself. The trade names and packing composition of various polysaccharide CSP columns are described in table 3. Figure 6 shows the structures of commonly used polysaccharide CSP.
Table 3: Polysaccharide-based chiral columns
Cyclodextrin chiral stationary phases
Cyclodextrin (CD) chiral stationary phase is produced by partial degradation of starch by the enzyme cyclodextrin glycosyltransferase followed by enzymatic coupling of the glucose units into a toroidal structure. These glucose units are connected through an α- (1,4) glycosidic linkages. The chair configuration of glucose residues makes the toroidal structure of CD molecule narrower at one end. Figure 7 shows the toroidal structure of the CD molecule [22]. The toroidal structure consists of a hydrophilic outer surface and a hydrophobic inner cavity. The exterior hydrophilic surface results from the secondary 2- and 3-hydroxyl groups lined at the mouth of the CD cavity and primary 6-hydroxyl groups found on the opposite end of the molecule. While the hydrophobic portion results from the glucose oxygens and methylene hydrogens.
CDs are cyclic oligosaccharides consisting of six (α CDs), seven (β CDs) and eight (γ CDs) glucopyranose units [12]. Figure 8 shows the structures of α, β, and γ cyclodextrin [23]. The chiral recognition mechanism is based on inclusion complexation. Complexation involves the interaction of the hydrophobic portion of an analyte molecule with the non-polar interior of the cavity, while the polar functional groups can form a hydrogen bond with the polar hydroxyl cavity opening. The most important factor that determines whether the analyte molecule will fit into the cyclodextrin cavity is its size. The α-CD consists of 30 stereogenic centers, β-CD consists of 35 stereogenic centers and γ-CD consists of 40 stereogenic centers. If the hydrophobic portion of the analyte is much larger or smaller than the cavity opening, inclusion will not occur. Table 4 lists some of the commercially available cyclodextrin columns.
Table 4: Commercially available cyclodextrin columns
|
Trade Name |
Support |
|
Cyclobond I 2000 |
β- Cyclodextrin |
|
Cyclobond II 2000 |
γ- Cyclodextrin |
|
Cyclobond III 2000 |
α- Cyclodextrin |
|
Cyclobond I 2000 Ac |
β-cyclodextrin, peracetylated |
|
Cyclobond I 2000 SP |
β-cyclodextrin, S-hydroxypropyl ether |
|
Cyclobond I 2000 RSP |
β-cyclodextrin, R,S-hydroxypropyl ether |
|
Cyclobond I 2000 RN |
β-cyclodextrin, R-Naphthylethyl carbamate |
|
Cyclobond I 2000 SN |
β-cyclodextrin, S-Naphthylethyl carbamate |
|
Cyclobond I 2000 DMP |
β-cyclodextrin, 3,5-Dimethylphenyl carbamate |
|
Chiral CD-PH |
phenylcarbamated-ß-cyclodextrin |
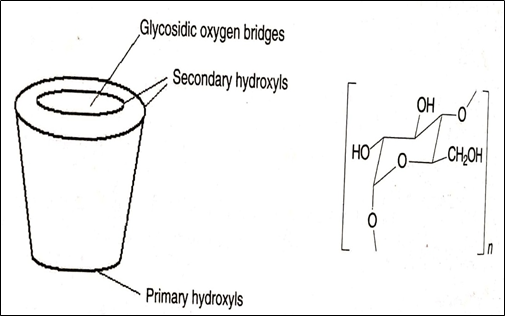
Fig.7. Toroidal structure of cyclodextrin molecule [22]
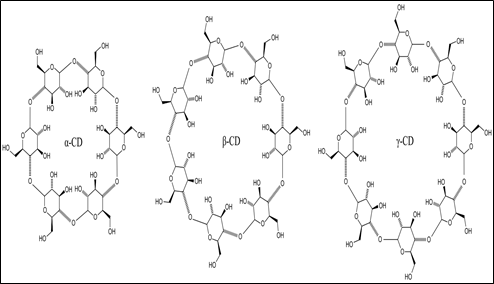
Fig.8. Chemical structure of α, β and γ cyclodextrin [22]
Ligand exchange chiral stationary phase
Ligand exchange chromatography was introduced by Davankov and Rogozhin. The principle involves the formation of a ternary mixed metal complex between the chiral selector, the analyte and a transition metal such as copper ions that will be at the core of the complex with the enantiomers. The lone electron pairs of the heteroatoms (N, O, S) of the functional groups, belonging to the analyte and selector, occupy definite positions in the coordination sphere of the central metal ion, to result in the formation of the ternary complex [24]. The amino acids act as a chiral selector (ligand). The chiral selector can either be fixed to the stationary phase or added to the mobile phase. In chiral ligand-exchange chromatography, the separation occurs as a result of the exchange of the ligand and the enantiomers on a metal ion. The ligand exchange involves the breaking and formation of coordinate bonds among the metal ions of the complex, ligands, and enantiomers. Therefore, ligand-exchange chromatography is useful for the chiral resolution of molecules containing electron-donating atoms such as oxygen, nitrogen, and sulfur [19]. Table 5 lists various ligand exchange-based commercial columns.
Table 5: Ligand exchange-based commercial columns
|
Trade Name |
Chiral Selector |
|
Chiralpak WH |
L-proline derivatives covalently bonded to silica gel |
|
Chiralpak MA (+) |
N,N-dioctyl-L-alanine coated on silica gel |
|
Nucleosil Chiral-1 |
L-hydroxyproline |
Macrocyclic chiral stationary phases
Macrocyclic antibiotics based chiral stationary phases were introduced by Armstrong in 1994. The commonly used macrocyclic antibiotics include rifamycin, glycopeptides (e.g. avoparcin, teicoplanin, ristocetin A, vancomycin, and their analogs), polypeptide antibiotic thiostrepton, and aminoglycosides (e.g. fradiomycin, kanamycin, and streptomycin). Figure 9 shows the subdivision of macrocyclic antibiotics [25]. Macrocyclic glycopeptides are complex molecules produced by microorganisms in the fermentation broth. The glycopeptides chiral stationary phase is made up of an aglycone portion of fused macrocyclic rings that forms a hydrophobic basket shape, which can include hydrophobic parts of an analyte and a carbohydrate moiety. The macrocyclic antibiotics interact with the analyte through hydrogen bonds, dipole-dipole interactions with the polar groups of the analyte, ionic interactions and π-π interactions. Figure 10 shows the structures of antibiotics used in the preparation of macrocyclic antibiotic CSP [14]. Table 6 lists commercially available macrocyclic antibiotic CSPs.
Table 6: Commercially available macrocyclic chiral stationary phase.
|
Trade Name |
Bonded Macrocyclic Glycopeptide |
|
Chirobiotic V, Chirobiotic V2 |
Vancomycin |
|
Chirobiotic T, Chirobiotic T2 |
Teicoplanin |
|
Chirobiotic R |
Ristocetin A |
|
Chirobiotic TAG |
Teicoplanin aglycone |
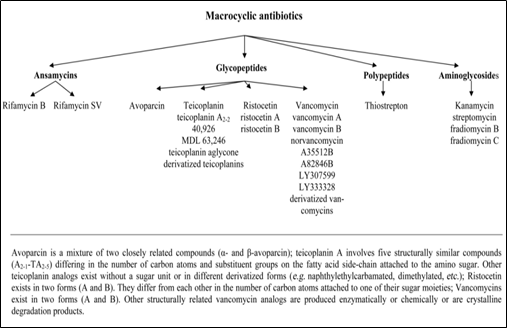
Fig. 9. Subdivision of macrocyclic antibiotics [25]
|
Antibiotic |
Structure |
|
Vancomycin |
|
|
Teicoplanin |
|
|
Teicoplanin Aglycone |
|
|
Ristocetin A |
|
|
Thiostrepton |
|
|
Rifamycin |
|
|
Fradiomycin |
|
|
Kanamycin |
|
|
Streptomycin |
 |
|
Avoparcin |
|
Fig. 10. Chemical structure of antibiotics used to prepare macrocyclic antibiotic chiral stationary phase [14]
Chiral crown ether
Crown ether was first developed by Charles Pederson in 1967. These are macrocyclic polyethers that form host-guest complexes with alkali, earth-alkali metal ions and ammonium cations [24]. The main skeleton in the cyclic structure consists of oxygen and methylene groups alternatively placed. The ether oxygens, which are electron donors, remain in the inner wall of the crown cavity and are surrounded by methylene groups in a collar fashion [19]. Two different diastereomeric inclusion complexes are formed. The primary interactions for complexation are hydrogen bonds between the three amine hydrogens and the oxygens of the macrocyclic ether in a tripod arrangement. Ionic, dipole-dipole interactions or hydrogen bonds between the carbocyclic groups and polar groups of the analytes may act as additional supporting interactions [26]. Figure 11 shows the chemical structure of a chiral crown ether [19]. The CROWNPAK CR (-) and CROWNPAK CR (+) columns are composed of chiral crown ether coated on silica-gel.
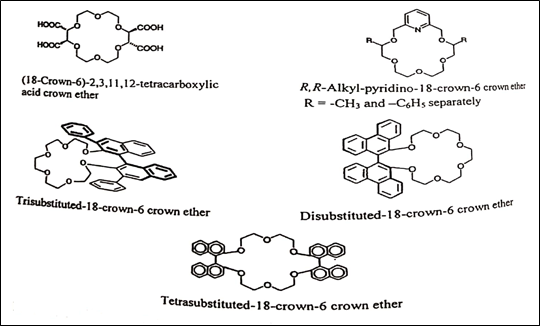
Fig. 11. Chemical structures of the chiral crown ethers [19]
Miscellaneous
Chiral resolution of some racemic compounds has also been reported on other CSPs such as alkaloid-based CSP, amide and amine-based CSP, acid-based CSP, and synthetic polymer-based CSP.
APPLICATIONS
Table 7 lists some chiral compounds separated using the different types of CSPs.
Table 7: Applications of chiral stationary phases
|
Sr. No. |
Chiral Compound |
Separation Technique |
Column |
Ref. No. |
|
1 |
S-(-) and R-(+) Metoprolol |
Chiral LC-ESI-MS/MS |
Chiral Lux amylose-2 |
[2] |
|
2 |
Acetyl-glutamine enantiomers |
HPLC-MS |
Chiralpak AD-H |
[8] |
|
3 |
Pyrrolidine derivatives |
Supercritical fluid chromatography |
Lux Cellulose-2 and Lux i- Cellulose-5 |
[27] |
|
4 |
Letermovir |
HPLC |
Chiralpak AD |
[18] |
|
5 |
Metolazone |
Liquid Chromatography |
Chiralpak AD-H |
[28] |
|
6 |
Atenolol, Metoprolol, Propranolol and Bisoprolol |
HPLC |
Chiralpak AD-H |
[29] |
|
7 |
Ezetimibe and Tramadol |
HPLC |
Chiralpak AS-H |
[30] |
|
8 |
Ketorolac |
RP-HPLC |
Chiral AGP |
[15] |
|
9 |
(S)-Tenofovir |
RP-HPLC |
Chiral AGP |
[31] |
|
10 |
Citalopram and Escitalopram |
HPLC |
Chiral CD-PH |
[32] |
|
11 |
(-)(R)-and (+)(S)-Chlorpheniramine and its metabolites |
HPLC |
CYCLOBOND I 2000TM |
[33] |
|
12 |
Benidipine Enantiomers |
LC-MS |
Chirobiotic V |
[9] |
|
13 |
Acetyl-L-carnitine |
LC |
SUMICHIRAL OA-6100 |
[34] |
CONCLUSION
Efficient separation of chiral drugs is critical to eliminate the differences in pharmacological as well as pharmacokinetic and pharmacodynamic effects. Chiral chromatography is a preferred method for the resolution of chiral drugs. Today, innovation in the chiral stationary phases has made the detection of enantiomers more effective.
Abbreviations:
CSP- Chiral stationary phase, CMPA- Chiral mobile phase additive, CAD- Chiral derivatising agent,
REFERENCES